-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- Advanced Training
-
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?






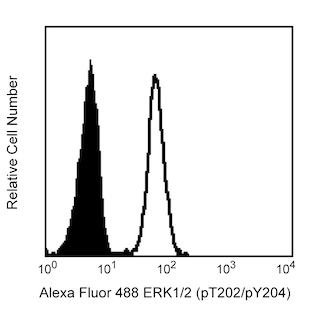
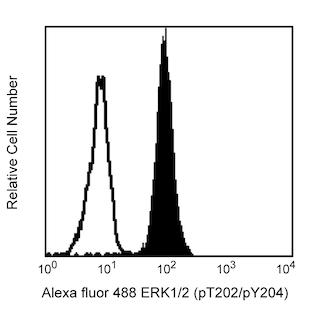
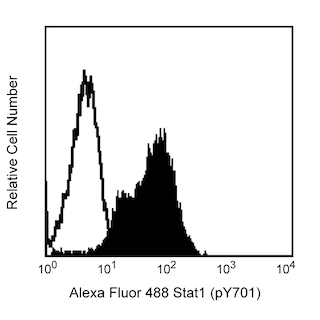
Flow cytometric analysis of Bcl-6 expression on Human Ramos (Left Panel). Ramos cells were fixed with BD Cytofix™ Fixation Buffer (Cat. No. 554655) and permeabilized with BD Phosflow™ Perm Buffer III (Cat. No. 558050), followed by intracellular staining with either PerCP-Cy™5.5 Mouse anti-Human Bcl-6 antibody (Cat. No. 562198, solid line histogram) or a PerCP-Cy™5.5 mIgG1, κ isotype control (Cat. No. 550795; dashed line histogram). Flow cytometric fluorescence histograms were derived from gated events with the forward and side light-scatter characteristics of viable cells. Flow cytometry was performed using a BD LSR™ II Flow Cytometry System.

Multicolor flow cytometric analysis of Bcl-6 expression on Mouse B lymphocytes (Middle and Right Panel). BALB/c mesenteric lymph node cells were stained with APC Rat Anti-Mouse B220 (Cat. No. 553092/561880), Alexa Fluor® 647 Rat Anti-Mouse CD4 (Cat. No. 557956/561025), FITC Hamster Anti-Mouse Fas/CD95 (Cat. No. 554257), and BD Horizon™ V450 Rat Anti-Mouse IgD (Cat. No. 560869). Cells were washed, resuspended in RPMI with 10% FBS, and fixed with BD Phosflow™ Lyse/Fix Buffer (Cat. No. 558049). Cells were permeabilized with BD Phosflow™ Perm/Wash Buffer I (Cat. No. 557885), followed by intracellular staining with PerCP-Cy™5.5 Mouse Anti-Human Bcl-6 (Cat. No. 562198). The two-color flow cytometric dot plot shows the expression of IgD versus Fas/CD95 by B cells identified as CD4-B220+ from gated events with the forward and side light-scatter characteristics of intact lymphocytes (Middle Panel). Germinal center B cells were identified as IgDloFas+ B lymphocytes. Flow cytometric fluorescence histograms (Right Panel) show intracellular Bcl-6 staining of mouse germinal center B cells (solid line histogram) and non-GC B cells (dashed line histogram). Flow cytometry was performed using a BD LSR™ II Flow Cytometry System.


BD Pharmingen™ PerCP-Cy™5.5 Mouse Anti-Bcl-6

BD Pharmingen™ PerCP-Cy™5.5 Mouse Anti-Bcl-6

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
We validate the quality of each batch of the K112-91 antibody conjugate by flow cytometry on human cell lines. Investigators may use the same cell lines as controls for their staining procedure, namely Ramos (Positive; ATCC CRL-1596) and Jurkat (Negative; ATCC TIB-152) human cell lines actively growing in log phase (do not overgrow). Cells are fixed with BD Cytofix™ Fixation Buffer (Cat. No. 554655; 10 minutes at 37°C), permeabilized with BD Phosflow™ Perm Buffer III (Cat. No. 558050; 30 minutes on ice), and washed using BD Pharmingen™ Stain Buffer (Cat. No. 554656), followed by intracellular staining with Mouse anti-Bcl-6 for 45 minutes at room temperature.
Product Notices
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- An isotype control should be used at the same concentration as the antibody of interest.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Cy is a trademark of Amersham Biosciences Limited. This conjugated product is sold under license to the following patents: US Patent Nos. 5,486,616; 5,569,587; 5,569,766; 5,627,027.
- Please observe the following precautions: Absorption of visible light can significantly alter the energy transfer occurring in any tandem fluorochrome conjugate; therefore, we recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to prevent exposure of conjugated reagents, including cells stained with those reagents, to room illumination.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- PerCP-Cy5.5–labelled antibodies can be used with FITC- and R-PE–labelled reagents in single-laser flow cytometers with no significant spectral overlap of PerCP-Cy5.5, FITC, and R-PE fluorescence.
- PerCP-Cy5.5 is optimized for use with a single argon ion laser emitting 488-nm light. Because of the broad absorption spectrum of the tandem fluorochrome, extra care must be taken when using dual-laser cytometers, which may directly excite both PerCP and Cy5.5™. We recommend the use of cross-beam compensation during data acquisition or software compensation during data analysis.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- This product is subject to proprietary rights of Amersham Biosciences Corp. and Carnegie Mellon University and made and sold under license from Amersham Biosciences Corp. This product is licensed for sale only for research. It is not licensed for any other use. If you require a commercial license to use this product and do not have one return this material, unopened to BD Biosciences, 10975 Torreyana Rd, San Diego, CA 92121 and any money paid for the material will be refunded.
- This product is sold under license to the following patent: US Patent No. 6,174,997.
Companion Products



The K112-91 monoclonal antibody specifically binds to Bcl-6. Bcl-6 was first identified as a proto-oncogene frequently deregulated by chromosomal translocations in non-Hodgkin B-cell lymphomas. It is a nuclear transcriptional repressor of the BTB/POZ zinc-finger family of transcription factors. In addition to its roles in cancer, Bcl-6 plays important roles in the differentiation of normal cells including B cells, thymocytes, CD4+ or CD8+ T cells. Bcl-6 is highly expressed in germinal center B cells, where it promotes the germinal center reaction by inducing proliferation and inhibiting the DNA-damage response. Bcl-6 has been identified as a key factor in promoting the differentiation of CD4+ follicular T helper (Tfh) cells that are involved in promoting germinal center formation and providing help to B cells. The interplay of Bcl-6 and another transcriptional repressor, Blimp-1, is thought to be critical in defining the results of both B-cell and T-cell differentiation.
Development References (11)
-
Baumjohann D, Okada T, Ansel KM. Cutting Edge: Distinct Waves of BCL6 Expression during T Follicular Helper Cell Development. J Immunol. 2011; 187(5):2089-2092. (Clone-specific: Flow cytometry). View Reference
-
Chung Y, Tanaka S, Chu F, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011; 17(8):983-988. (Clone-specific: Flow cytometry). View Reference
-
Crotty S, Choi YS, Kageyama R, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011; 34:1-15. (Clone-specific: Flow cytometry). View Reference
-
Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010; 11(2):114-120. (Biology). View Reference
-
Crotty S. Follicular Helper CD4 T Cells (Tfh). Annu Rev Immunol. 2011; 29(1):621-663. (Biology). View Reference
-
Eto, D., C. Lao, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE. 2011; 6(3):e17739. (Clone-specific: Flow cytometry). View Reference
-
Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Rev Immunol. 2009; 10(4):375-384. (Biology). View Reference
-
Johnston RJ, Poholek AC, DiToro D, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation.. Science. 2009; 325(5943):1006-10. (Biology). View Reference
-
Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008; 8(1):22-33. (Biology). View Reference
-
Nurieva RI, Chung Y, Martinez GJ, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009; 325(5943):1001-1005. (Biology). View Reference
-
Ye BH, Lista F, Lo Coco F, et al. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science. 1993; 262(5134):747-750. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.