-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- Advanced Training
-
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Phosflow™ T Cell Kit Lyophilized Cells
(RUO)


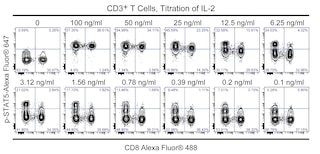
BD Phosflow analysis of Treated and Untreated Human Control Cells for phosphorylated signal molecules expressed in CD4+ and CD8+ T cells. The lyophilized BD Phosflow™ Treated and Untreated Human Control Cells (6 vials of each) were reconstituted, stained, washed, resuspended, and analyzed as described in the Recommended Assay Procedure. All tubes were stained with the BD Phosflow™ Human T-Cell (CD4/CD8) Antibody Cocktail (containing Alexa Fluor® 488 anti-Human CD8, PE anti-Human CD3, and PerCP-Cy™5.5 anti-Human CD4 antibodies). Then Alexa Fluor® 647-conjugated Mouse anti-Human antibodies specific for ERK1/2 (pT202/pY204) (Cat. No. 612593), p38 MAPK (pT180/pY182) (Cat. No. 612595), Stat1 (pY701) (Cat. No. 612597), Stat3 (pY705) (Cat. No. 557815), Stat5 (pY694) (Cat. No. 612599), or Stat6 (pY641) (Cat. No. 612601) were added individually to each tube. The antibody cocktail and phosphorylation site-specific antibodies are components of the BD Phosflow™ T Cell Activation Kit (Cat. No. 560750). The overlapping fluorescence histograms (Panel B. Activation Profiles) of phosphorylated signal molecule expression for Untreated (red line histograms) and Treated (blue line histograms) cells were derived from events with the forward and side light-scatter and staining characteristics of intact CD4+ and CD8+ T lymphocytes (Panel A. Gating Strategy). Flow cytometry was performed using a BD FACSCanto™ II Flow Cytometer System.

BD™ Phosflow T Cell Kit Lyophilized Cells
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
1. Gently tap the vial on a flat surface before using. This ensures that the lyophilized cell pellet is on the bottom of the vial.
2. Open a vial of BD Phosflow™ Treated or Untreated Human Control Cells. Reconstitute the cells in 120 uL of BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) at room temperature. Gently vortex the cells for 1 to 2 seconds and let stand for at least 5 minutes before use.
3. Transfer 100 µL of control cells to a 12 × 75-mm polystyrene tube. Keep cells on ice until ready to stain. Add fluorescent antibodies specific for intracellular signaling proteins and cell surface antigens (ie, fluorescent antibodies listed in the figure legend that have been validated for these cells), vortex the tube, and incubate at room temperature for 1 hour, protected from light.
4. Wash cells by adding 2 mL of Stain Buffer and immediately centrifuging them (600g for 6 min), aspirate the supernatant, and resuspend the stained cells in 300 to 500 µL of Stain Buffer. Analyze the cells by flow cytometry immediately (optimal) or no longer than 4 hours after preparation. Keep the cells at 2 to 8°C and protected from light prior to data acquisition.
Product Notices
- This product contains human blood, serum, cells, or materials derived from them, which are potentially hazardous materials. Use universal precautions when handling. Handle as if product were capable of transmitting disease. Material used in this product has been tested using FDA approved methods and found negative for Human Immunodeficiency Virus (HIV-1/HIV-2), Hepatitis B Surface Antigen (HBSAG) and antibody to Hepatitis C Virus (HCV). However, no known test method can offer complete assurance that specimens of human origin will not transmit infectious disease. When handling or disposing, follow precautions described in CDC and FDA recommendations and OSHA Bloodborne Pathogen recommendations.
- Product Use Limitations: This product is for research use only. Research products are labeled as Research Use Only (RUO) and are not for use in diagnostic or therapeutic procedures. Customer agrees not to use the product for purposes which allow for any identification of the individual donors. Customer agrees to notify BDB Technical Services within five (5) business days of becoming aware of any identification or re-identification of an individual in connection with this product.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products

Component: 51-9006610A
Description: Treated Human Control Cells - T Cells
Size: 1 test per vial (5 EA)
Component: 51-9006610B
Description: Untreated Human Control Cells - T Cells
Size: 1 test per vial (5 EA)
The BD Phosflow™ T Cell Kit Lyophilized Cells contains five vials each of lyophilized Treated and Untreated Human Control Cells. Each vial of control cells contains fixed, permeabilized, and lyophilized human peripheral blood leukocytes (PBL). The cells are intended to serve as biological controls for antibody staining in BD Phosflow studies designed to analyze cell signaling events associated with T-cell activation. These events include the phosphorylation status of signaling molecules that mediate the intracellular MAPK and JAK/STAT signaling pathway responses transduced by stimulated human CD4+ and CD8+ T cells.
The BD Phosflow™ Treated Human Control Cells - T cells are PBL that were stimulated to express easily detectable intracellular levels of several phosphorylated signaling molecules including the serine/threonine kinases, Erk1/2 (p44/p42 MAPK) and p38 MAPK and Signal Transducers and Activators of Transcription (STAT) proteins, Stat1, Stat3, Stat5, and Stat6 as determined by multicolor BD Phosflow™ analysis. The treated control cells were activated with phorbol 12-myristate 13-acetate (PMA), interferon-alpha (IFN-α), interleukin-2 (IL-2), IL-4, and IL-6. These activators are known to induce the cellular phosphorylation of the specified molecules that are often targeted in T cell signaling studies. Each vial of BD Phosflow™ Untreated Human Control Cells - T cells contains PBL from the same donor as the treated cells. These control cells were cultured without activators and express background levels of the phosphorylated target signal proteins. The Treated and Untreated Control Cells were fixed with BD Phosflow™ Lyse/Fix Buffer (Cat. No. 558049) and permeabilized with BD Phosflow™ Perm Buffer III (Cat. No. 558050). The cells were then washed with BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) and lyophilized. Each vial contains a sufficient number of Treated or Untreated Control Cells for one multicolor stain using fluorescent antibodies specific for CD3, CD4, and CD8 and a single phosphorylated target protein.
Each lot of Treated Control Cells contains a measurable proportion of cells that express the specified phosphorylated signaling proteins. Representative BD Phosflow results are shown for typical staining of CD4+ and CD8+ Treated and Untreated Control Cells. Data from individual lots of these control cells may differ due to donor variation. Investigators should anticipate similar (though not identical) results to those shown due to differences in staining methodology, fluorescent antibody reagents and flow cytometers or cytometer settings.
Development References (4)
-
Montag DT, Lotze MT. Rapid flow cytometric measurement of cytokine-induced phosphorylation pathways [CIPP] in human peripheral blood leukocytes. Clin Immunol. 2006; 121(2):215-226. (Methodology: Flow cytometry). View Reference
-
Montag DT, Lotze MT. Successful simultaneous measurement of cell membrane and cytokine induced phosphorylation pathways [CIPP] in human peripheral blood mononuclear cells. J Immunol Methods. 2006; 313(1-2):48-60. (Methodology: Flow cytometry). View Reference
-
Perez OD, Mitchell D, Campos R, Gao GJ, Li L, Nolan GP. Multiparameter analysis of intracellular phosphoepitopes in immunophenotyped cell populations by flow cytometry. Curr Protoc Cytom. 2005; 6.20.1-6.20.22. (Methodology: Flow cytometry). View Reference
-
Perez OD, Mitchell D, Jager GC, et al. Leukocyte functional antigen 1 lowers T cell activation thresholds and signaling through cytohesin-1 and Jun-activating binding protein 1. Nat Immunol. 2003; 4(11):1083-1092. (Methodology: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.