-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- Advanced Training
-
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Calcein AM
(RUO)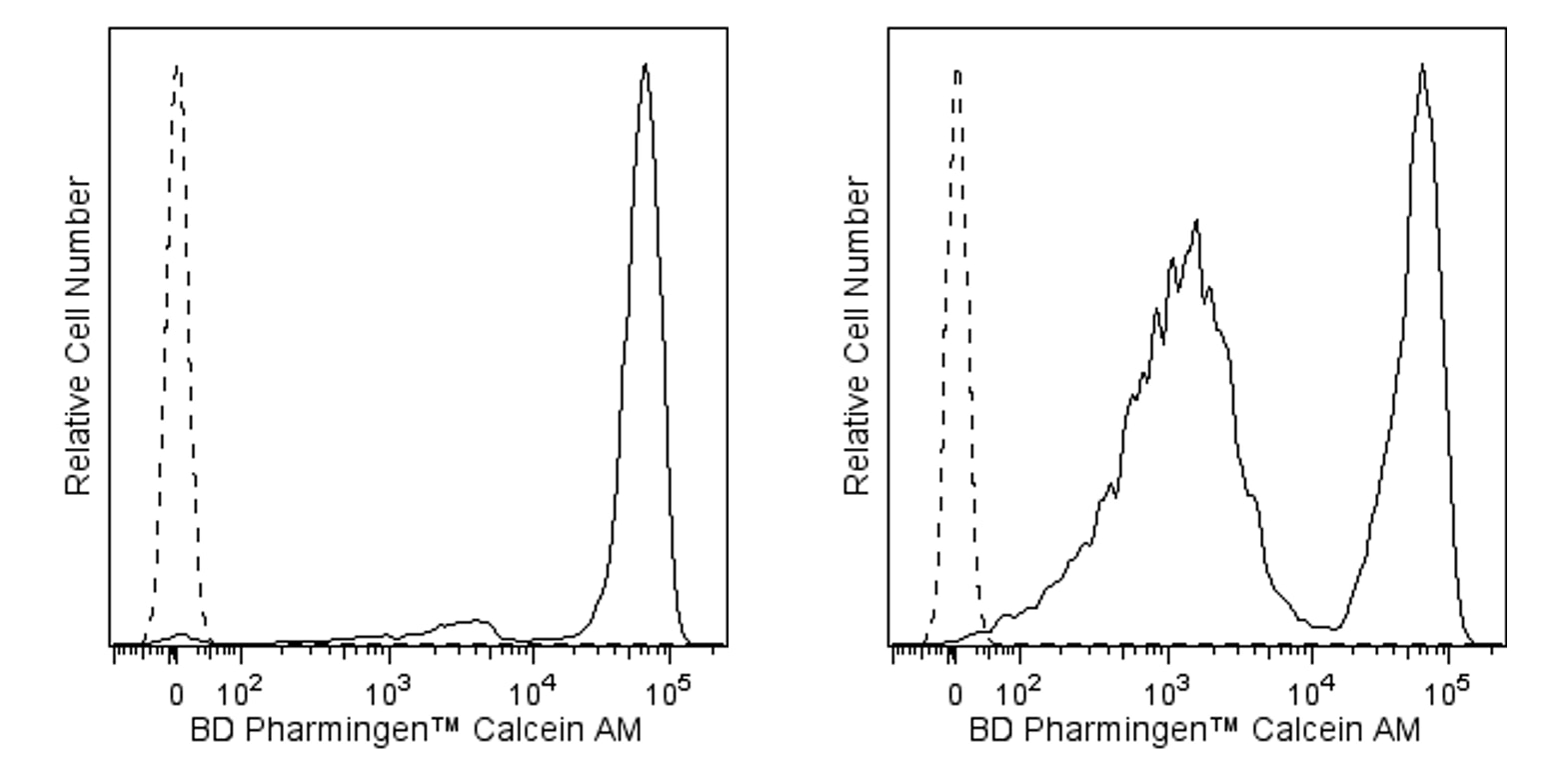



Flow cytometric analysis of BD Pharmingen™ Calcein AM fluorescence in Jurkat Cells. Cells from the human Jurkat (Acute T cell leukemia, ATCC TIB-152) cell line were treated with 0.025% DMSO (Left Panel) or 5 μM camptothecin (Right Panel) for 20 hours and then stained with 10 μM BD Pharmingen™ Calcein AM (Cat. No. 564061; solid line histograms) in serum-free buffer. Unstained cells are indicated by the dashed line histograms. Non-viable cells show a decrease in fluorescence intensity. Histograms were derived from gated events with the light scattering characteristics of Jurkat cells. Flow cytometric analysis was performed using a BD LSRFortessa™ Cell Analyzer System. Calcein AM has also been tested on mouse (data not shown).

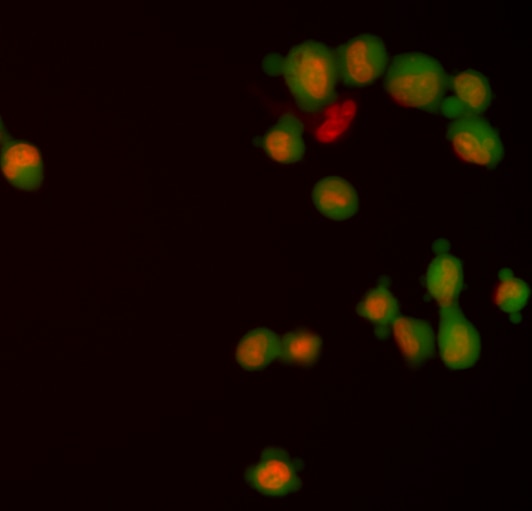
Fluorescent staining of human U-2 OS cells with BD Pharmingen™ Calcein AM. Cells from the U-2 OS (Osteosarcoma, ATCC HTB-96) cell line were stained with 10 μM BD Pharmingen™ Calcein AM (pseudo-colored green) in serum-free buffer. The cells were counterstained with DRAQ5™ (Cat. No. 564903/564902)(pseudo-colored red). The images were captured using a BD Pathway™ 435 Cell Analyzer and merged using BD AttoVision™ Software.

BD Pharmingen™ Calcein AM

BD Pharmingen™ Calcein AM
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Recommended Assay Procedures
Preparation
Bring BD Pharmingen™ Calcein AM powder and fresh cell culture-grade Dimethyl Sulfoxide (DMSO; e.g., Sigma, Cat. No. D2650) to room temperature. Add 201 μl of DMSO to make a 5 mM stock solution (Molar Mass = 994.86 g/mol) and vortex solution well. Inspect the solution and repeat vortex until the stock dye has fully dissolved.
Storage
Store BD Pharmingen™ Calcein AM desiccated at -80°C until use. After reconstitution with DMSO, store the solution at -20°C in small aliquots. Avoid freeze-thaw cycles. Please discard the dye aliquots after 6 months post reconstitution with DMSO.
Notes
• When diluted with an aqueous solution, BD Pharmingen™ Calcein AM should be used immediately after dilution, as the esterase-cleavable dye may spontaneously hydrolyze with prolonged aqueous exposure.
• Please note that these dyes are esterase cleavable. Serum may have esterase activity, and serum-containing buffers may decrease the resolution of viable and non-viable cells if used during staining. Serum-containing buffers may be used for wash steps after staining is complete.
• The spillover properties of this dye are similar to FITC. As such, we recommend appropriate compensation for other colors excited by the blue laser, such as PE or PerCP-Cy™5.5. Additionally, we recommend using cells stained with BD Pharmingen™ Calcein AM for compensation.
• This dye is not fixable.
General Procedure
1. Prepare cells for flow cytometry staining using serum-free buffer (e.g., 1X Dulbecco's Phosphate Buffered Saline).
2. Wash cells one time in serum-free buffer.
3. Resuspend cells at ~1-10 x 10^6 cells/ml in dye diluted in serum-free buffer.
a. We recommend staining at a 1-10 μM final concentration of BD Pharmingen™ Calcein AM dye for live/dead cell discrimination, or at 0.1-1 μM for multicolor applications as a starting point. Since fluorescence intensity may vary based on cell type and experimental conditions, a titration is recommended to determine the optimal dye concentration for each experimental protocol.
4. Incubate the mixture for 30 minutes at room temperature protected from light.
5. Wash cells twice with 2 ml of BD Pharmingen™ Stain Buffer (FBS) or the equivalent.
6. Aspirate the supernatant and gently mix to disrupt the cell pellet.
7. Resuspend the cells in Stain Buffer (FBS) or equivalent.
8. Analyze by flow cytometry or stain cells as desired for downstream applications.
Notes on Adherent Cells
Adherent cells may be stained in suspension or in situ.
To stain adherent cells in suspension, follow the "General Procedure" as outlined above after cell dissociation into a single cell suspension. Note that for some cells, enzymatic removal from the growth substrate may impact cell membrane integrity, which may affect staining with BD Pharmingen™ Calcein AM. Allowing the cells to recover from the enzymatic dissociation in growth media is recommended. The time of recovery may vary by cell line, so it may be useful to test multiple recovery periods in initial experiments.
To stain in situ, we recommend the following protocol:
1. Remove media and wash the cells once with serum-free buffer to remove any remaining media.
2. Add dye diluted in serum-free buffer to the culture.
a. We recommend staining at a 1-10 μM concentration of BD Pharmingen™ Calcein AM dye for live/dead cell discrimination, or at 0.1-1 μM for multicolor applications. Since brightness may vary based on cell type and experimental conditions, a titration is recommended to determine the optimal dye concentration for each experimental protocol.
3. Incubate the mixture for 30-60 minutes at 37°C protected from light.
4. Wash cultures twice with BD Pharmingen™ Stain Buffer (FBS) or the equivalent.
5. Remove the cells from the growth substrate. We recommend using BD™ Accutase™ Cell Detachment Solution.
a. Wash cells twice with BD Pharmingen™ Stain Buffer (FBS) or the equivalent.
b. Resuspend the cells in Stain Buffer (FBS) or equivalent.
c. Analyze by flow cytometry or stain cells as desired for downstream applications.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Accutase is a registered trademark of Innovative Cell Technologies, Inc.
- All other brands are trademarks of their respective owners.
- Cy is a trademark of GE Healthcare.
- Before staining with this reagent, please confirm that your flow cytometer is capable of exciting the fluorochrome and discriminating the resulting fluorescence.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products




BD Pharmingen™ Calcein AM is used for labeling live cells that can be detected and further analyzed by flow cytometry or fluorescence imaging. The enhanced hydrophobicity of the acetomethoxy (AM) derivative of Calcein allows this dye to readily enter viable cells. Once inside, intracellular esterases cleave the AM groups off of the non-fluorescent BD Pharmingen™ Calcein AM, trapping fluorescent Calcein within the cell. Since dead cells lack esterase activity, only viable cells are labeled. Calcein AM is optimally excited at the 495 nm wavelength of light and emits maximally at 515 nm.
Development References (4)
-
Coder DM. Assessment of cell viability. Curr Protoc Cytom. 2013; Ch. 9(Unit 9.2):1-26. (Methodology). View Reference
-
Gatti R, Belletti S, Orlandini G, Bussolati O, Dall'Asta V, Gazzola GC. Comparison of annexin V and calcein-AM as early vital markers of apoptosis in adherent cells by confocal laser microscopy. J Histochem Cytochem. 1998; 46(8):895-900. (Biology). View Reference
-
Giordano G, Hong S, Faustman EM, Costa LG. Measurements of cell death in neuronal and glial cells. Methods Mol Biol. 2011; 758:171-178. (Biology). View Reference
-
Huitink GM, Poe DP, Diehl H. On the properties of Calcein Blue. Talanta. 1974; 21(12):1221-1229. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.