-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- Advanced Training
-
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Caspase-3 Assay Kit
(RUO)



Spectrofluorometric Analysis of Caspase-3 Activity. Lysates were prepared from Daudi B cells that were either untreated (left panel) or treated with Purified NA/LE Mouse Anti-Human CD95 (Cat. No. 555670) and Protein G (middle and right panels) for 4 hours. Cell lysates were incubated with the Ac-DEVD-AMC, caspase-3 substrate alone (left panel) or with the substrate and the Ac-DEVD-CHO inhibitor (right panel) and analyzed by spectrofluorometry. Left panel: Lysates from untreated cells did not emit fluorescence, indicating that the substrate was not cleaved and hence caspase-3 activity was absent. Middle panel: Lysates from cells treated with anti-Fas mAb cleaved the substrate, indicating the presence of caspase-3 activity. Right panel: Fluorescence was not emitted in lysates from cells treated with anti-Fas mAb when both the inhibitor and substrate were added, indicating that caspase-3 activity was blocked. The addition of Protein G enhances the ability of DX2 to induce apoptosis, presumably by cross-linking Fas receptors.


BD Pharmingen™ Caspase-3 Assay Kit

Caspase-3 Assay Kit
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
Component 51-6632AK consists of:
51-6635KC PBS Buffer (10X) 50 ml
51-6636KC Cell Lysis Buffer (1X) 50 ml
51-6637KC HEPES Buffer (2X) 50 ml
Component 51-6632BK consists of:
51-66081U Caspase-3 Fluorogenic Substrate 1 X 1.0 mg
N-acetyl-Asp-Glu-Val-Asp-AMC (7-amino-4-methylcoumarin)
51-66221U Caspase-3 Inhibitor 1 X 1.0 mg
Ac-DEVD-CHO [N-acetyl-Asp-Glu-Val-Asp-CHO (aldehyde)]
This kit is designed to measure caspase-3 or DEVD-cleaving activity, an early marker of cells undergoing apoptosis. The kit can also be used to determine if certain apoptosis pathways involve caspase-3 activation. It contains a caspase-3 fluorogenic substrate (Ac-DEVD-AMC), a caspase-3 aldehyde inhibitor (Ac-DEVD-CHO) and assay buffers.
Ac-DEVD-AMC is a synthetic tetrapeptide fluorogenic substrate (M.W. = 675 Daltons; purity ≥98%) that is used to identify and quantitate caspase-3 activity in apoptotic cell lysates. Ac-DEVD-AMC has been reported to have linear Michaelis-Menton kinetics with a Km of 10 µM for caspase-3. Caspase-3 cleaves the tetrapeptide between D and AMC, releasing the fluorescent AMC which can be quantified in cell lysates by ultraviolet (UV) spectrofluorometry using an excitation wavelength of 380 nm and an emission wavelength range of 420-460 nm. Apoptotic cell lysates containing active caspase-3 yield a considerable emission as compared to non-apoptotic cell lysates (Fig. 1).
Ac-DEVD-CHO is a potent, synthetic tetrapeptide inhibitor (M.W. = 502.5 Daltons; purity ≥98%) of caspase 3 activity. The inhibitor is used to block caspase-3 activity in apoptotic cells or cell lysates (Fig. 1). Ac-DEVD-CHO has been reported to have linear Michaelis-Menton kinetics with a Ki of less than 1 nM for Caspase-3. This Ki (1 nM) is significantly less than the Km of the Ac-DEVD-AMC substrate (10 µM). Thus, the amount of Ac-DEVD-CHO required for caspase-3 assays is much less than that of Ac-DEVD-AMC. When apoptotic cell lysates are incubated with both substrate and inhibitor, caspase-3 activity is blocked by Ac-DEVD-CHO. As a result, AMC is not released from Ac-DEVD-AMC and fluorescence is not emitted upon excitation of the samples.
Note: Investigators are advised that HEPES Buffer (2X) (Component 51-6637KC) can exhibit subtle color variation from clear to light yellow. This has been shown to not impact performance.
Preparation and Storage
Preparation And Storage
The Caspase-3 Kit contains two parts (A and B), which are shipped separately. Part A (Cat. No. 51-6632AK) is stored at 4°C and shipped at room temperature. Part B (Cat. No. 51-6632BK) is stored at -20°C and shipped on dry ice. We have determined that this shipping method is adequate to preserve kit integrity. Upon arrival, immediately store products at their appropriate storage conditions.
Recommended Assay Procedures
METHODS FOR SPECTROFLUOROMETRIC ANALYSIS OF CASPASE-3 ACTIVITY
Induction of Apoptosis
Apoptosis can be induced by a number of protocols, please refer to the literature for specifics of the particular biological model system you are working with. At BD Biosciences Pharmingen, we routinely induce apoptosis by using Fas (see Fig. 1) or camptothecin. For additional methods for measuring apoptosis please see BD PharMingen's Apoptosis Instruction Manual (please contact BD Biosciences Pharmingen customer service to request a copy of this manual).
Induction of Apoptosis in Daudi B Cells by Fas Materials:
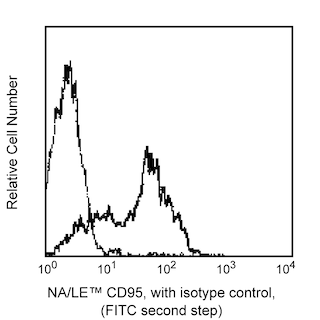
1. Purified NA/LE Mouse Anti-Human CD95 (Clone DX2, Cat. No. 555670)
2. Recombinant Protein G (Sigma, Cat. No. P4689)
3. A cell line or primary cells that can be easily induced to undergo apoptosis by human Fas mAb, such as Daudi (ATCC CCL-213), or Jurkat (ATCC TIB-152).
Procedure:
1. Maintain cells in culture and change the medium one day prior to inducing apoptosis.
2. Add 0.5-2.0 µg/ml of DX2 mAb and 1-2 µg/ml of Protein G to a T25 flask with medium containing approximately 1.0 x 10^6 cells/ml.
3. Incubate the cells for 2-12 hr at 37°C.
Induction of Apoptosis in Jurkat Cells by Camptothecin
Camptothecin (an extract of the Chinese tree Camptotheca acuminata) is a potent inhibitor of topoisomerase I, a molecule required for DNA synthesis. Camptothecin has been shown to induce apoptosis in a dose dependent manner in vitro. Camptothecin is used at PharMingen as a general method for inducing apoptosis.
Materials:
1. Prepare a 1.0 mM stock solution of camptothecin (SIGMA Cat. No. C-9911) in DMSO.
2. Jurkat T cells (ATCC TIB-152).
Procedure:
1. Add camptothecin (4-6 µM final concentration) to 1x10^6 /ml proliferating Jurkat cells.
2. Incubate the cells for 4 hr at 37°C.
MONITORING AMC RELEASE
The Caspase-3 Assay can be performed using 1.5 Eppendorf tubes or in a multiwell plate format.
Reagents
1. Caspase-3 Fluorogenic Substrate, Ac-DEVD-AMC (Component No. 51-66081U): 1 x 1.0 mg peptide; lyophilized. Store the lyophilized Ac-DEVD-AMC substrate at -20°C. Prior to use, reconstitute 1.0 mg of peptide in 1.0 ml DMSO to yield 1.0 mg/ml in DMSO. Aliquot and store the reconstituted substrate at -20°C, and avoid repeated freeze thaw cycles.
2. Caspase-3 Inhibitor, Ac-DEVD-CHO (Component No. 51-66221U): 1 x 1.0 mg peptide; lyophilized. Store the lyophilized Ac-DEVD-CHO inhibitor at -20°C. Prior to use, reconstitute 1.0 mg of peptide in 1.0 ml of DMSO to yield 1.0 mg/ml in DMSO. Reconstitute the inhibitor before use. Aliquot and store the reconstituted substrate at -20°C, and avoid repeated freeze thaw cycles.
3. PBS Buffer (10X), 50 ml (Component No: 51-6635KC): 1.4 M NaCl; 27 mM KCl; 100 mM KH2PO4/K2HPO4 (pH 7.5). Dilute to 1X with sterile distilled H2O prior to use. Adjust the pH to 7.5. Store the buffer at 4°C.
4. Cell Lysis Buffer (1X), 50 ml (Component No: 51-6636KC): 10 mM Tris-HCl; 10 mM NaH2PO4/NaHPO4 (pH 7.5); 130 mM NaCl; 1% Triton®-X-100; 10 mM NaPPi (sodium pyrophosphate); sterile filtered. Store the buffer at 4°C.
5. HEPES [Later referred to as "Protease Assay Buffer"] (2X), 50 ml (Component No: 51-6637KC): 40 mM HEPES (pH 7.5); 20% glycerol; 4 mM DTT. Dilute to 1X with sterile distilled H2O prior to use. Short term storage at RT. For long term storage, store at 4°C.
Note: Investigators are advised that HEPES Buffer (2X) (Component 51-6637KC) can exhibit subtle color variation from clear to light yellow. This has been shown to not impact performance.
EPPENDORF TUBE PROTOCOL
Procedure
1. Induce apoptosis in primary cells or in established cell lines.
2. Prepare cell lysates (1-10 x 10^6 cells/ml) at various time points after induction of apoptosis:
- For cells in suspension, pellet (1-10 x 10^6 cells/ml), wash with PBS, resuspend in cold Cell Lysis Buffer, and incubate 30 min on ice.
- For adherent cells, decant the media and wash rapidly with PBS. Remove excess PBS by aspiration and respend in cold Cell Lysis Buffer (1-10 x 10^6 cells/ml) for 30 min on ice.
3. For each reaction or time point, add 15 µl reconstituted Ac-DEVD-AMC to a tube containing 1 ml of 1X HEPES buffer (which will be referred to as Protease Assay Buffer).
4. Add an appropriate amount of cell lysate (50-100 µl of cell lysate) to each tube containing Protease Assay Buffer. The amount of apoptotic cell lysate required to cleave the Ac-DEVD-AMC will vary for each experimental system depending on the extent of apoptosis or caspase-3 activation and should be titrated. We generally titrate between 50-100 µl of cell lysate per reaction.
5. Incubate the reaction mixtures for 1 hr at 37°C.
6. Measure the amount of AMC liberated from Ac-DEVD-AMC using a spectrofluorometer with an excitation wavelength of 380 nm and an emission wavelength range of 420-460 nm. Apoptotic cell lysates containing active caspase-3 yield a considerable emission as compared to non-apoptotic cell lysates (Fig. 1).
MULTIWELL PLATE PROTOCOL
Procedure
1. Induce apoptosis in primary cells or in established cell lines.
2. Prepare cell lysates (1-10 x 10^6 cells/ml) at various time points after induction of apoptosis:
- For cells in suspension, pellet (1 -10 x 10^6 cell s/m l), wash with PBS, resuspend in cold Cell Lysis Buffer, and incubate for 30 min on ice.
- For adherent cells, decant the media and wash rapidly with PBS. Remove excess PBS by aspiration and respend in cold Cell Lysis Buffer (1-10 x 10^6 cells/ml) for 30 min on ice.
3. For each reaction or time point, add 5 µl reconstituted Ac-DEVD-AMC to a well containing 0.2 ml of 1X HEPES buffer (which will be referred to as Protease Assay Buffer).
4. Add an appropriate amount of cell lysate (10-50 µl) to each well/reaction. The amount of apoptotic cell lysate required to cleave the Ac-DEVD-AMC will vary for each experimental system depending on the extent of apoptosis or caspase-3 activation and should be titrated. We generally titrate between 10-50 µl of cell lysate per reaction.
5. Incubate the reaction mixtures for 1 hr at 37°C.
6. Measure the amount of AMC liberated from Ac-DEVD-AMC using a plate reader with an excitation wavelength of 380 nm and an emission wavelength range of 420-460 nm. Apoptotic cell lysates containing active caspase-3 yield a considerable emission as compared to non-apoptotic cell lysates (Fig. 1).
Suggested Controls
1. Reaction mixtures with Ac-DEVD-AMC only (no cell lysate). These reactions are used as negative controls.
2. Reaction mixtures with cell lysate only (no Ac-DEVD-AMC). These reactions are used as negative controls.
3. Reaction mixtures with non-apoptotic cell lysates and Ac-DEVD-AMC. These reactions are used to determine the basal level of apoptosis in cell populations. It is important to note that basal levels of apoptosis can vary considerably depending on the cell type.
Caspase-3 Blocking Assays for Multiwell Plate Procedure
For each blocking reaction add the following to each well:
1. 200 µl of Protease Assay Buffer
2. 5 µl reconstituted Ac-DEVD-AMC
3. 5 µl reconstituted Ac-DEVD-CHO
4. 10-50 µl of apoptotic cell lysate
Set up the reactions in parallel with Step 3 of the Multiwell Plate Procedure. Continue with Steps 5-6. Cell lysates incubated with both Ac-DEVD-AMC and Ac-DEVD-CHO should not emit fluorescence above the negative control.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
| Description | Quantity/Size | Part Number | EntrezGene ID |
|---|---|---|---|
| Caspase-3 Assay Kit Part A | N/A | 51-6632AK | N/A |
| Caspase-3 Assay Kit Part B | N/A | 51-6632BK | N/A |
Development References (1)
-
Nicholson DW, Ali A, Thornberry NA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995; 376(6535):17-18. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.