-
抗体試薬
- フローサイトメトリー用試薬
-
ウェスタンブロッティング抗体試薬
- イムノアッセイ試薬
-
シングルセル試薬
- BD® AbSeq Assay | シングルセル試薬
- BD Rhapsody™ Accessory Kits | シングルセル試薬
- BD® Single-Cell Multiplexing Kit | シングルセル試薬
- BD Rhapsody™ Targeted mRNA Kits | シングルセル試薬
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit | シングルセル試薬
- BD Rhapsody™ TCR/BCR Profiling Assays (VDJ Assays) | シングルセル試薬
- BD® OMICS-Guard Sample Preservation Buffer
-
細胞機能評価のための試薬
-
顕微鏡・イメージング用試薬
-
細胞調製・分離試薬
-
- BD® AbSeq Assay | シングルセル試薬
- BD Rhapsody™ Accessory Kits | シングルセル試薬
- BD® Single-Cell Multiplexing Kit | シングルセル試薬
- BD Rhapsody™ Targeted mRNA Kits | シングルセル試薬
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit | シングルセル試薬
- BD Rhapsody™ TCR/BCR Profiling Assays (VDJ Assays) | シングルセル試薬
- BD® OMICS-Guard Sample Preservation Buffer
- Japan (Japanese)
-
Change country/language
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
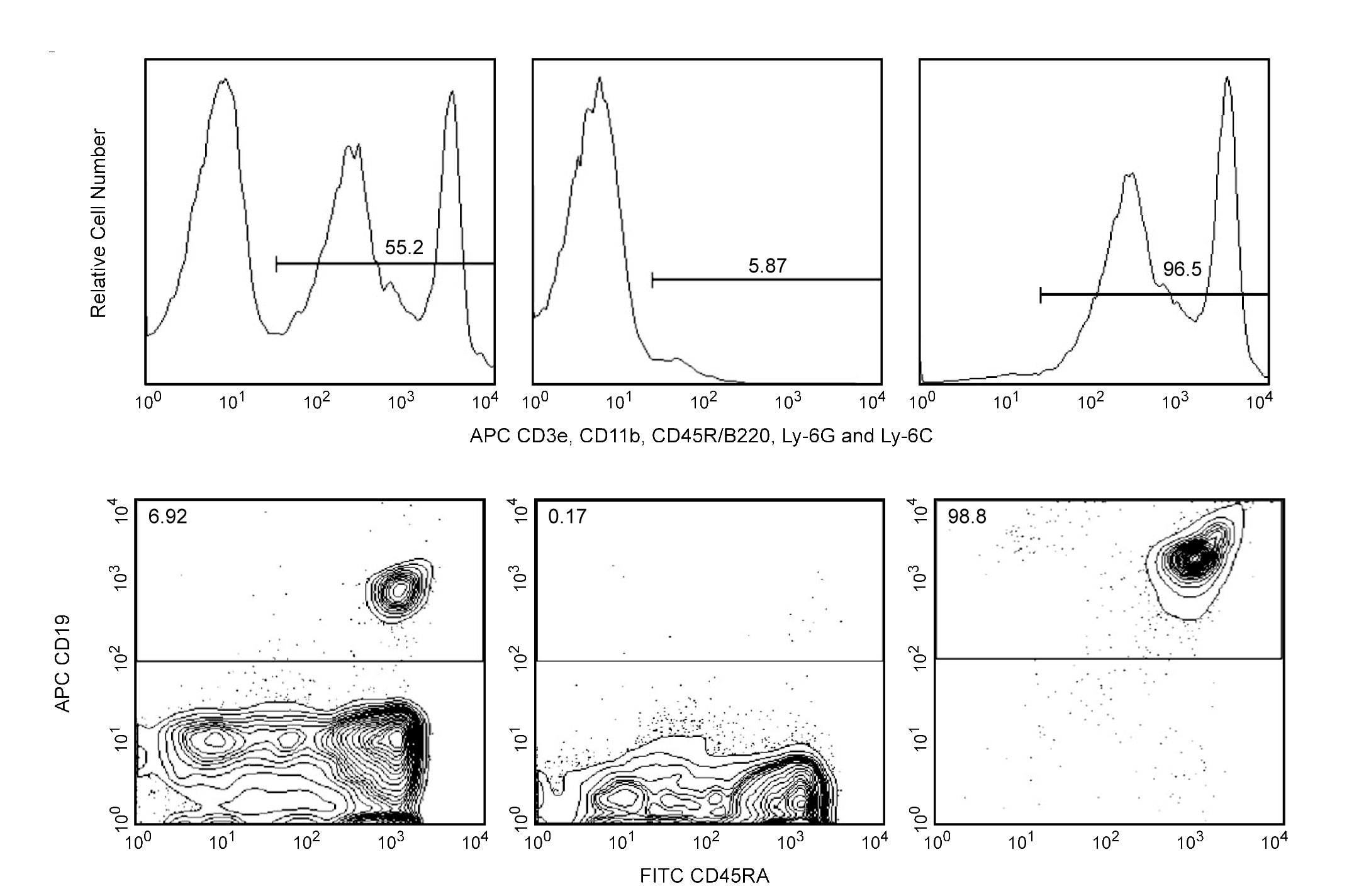



Depletion of T-, B-, NK-, and myeloid-lineage cells from mouse bone marrow (top panels). BALB/c bone-marrow cells were labeled with APC Anti-Mouse CD3e (Cat. No. 553066), CD11b (Cat. No. 553312), CD45R/B220 (Cat. No. 553092), and Ly-6G and Ly-6C (Cat. No. 553129) followed by BD IMag™ Anti-APC Particles - DM (Cat. No.557932) according to the depletion protocol. The labeled cells were separated using the BD IMag™ Cell Separation Magnet (Cat. No. 552311). Please refer to the Depletion Flow Chart to identify the separated cell populations represented in this figure. Unseparated bone-marrow cells (left panel), the final depleted fraction (middle panel), and the positive fraction (right panel) were analyzed by flow cytometry. The percentage of positive cells is indicated in each panel; placement of each marker is based upon staining with the appropriate isotype control (data not shown). Flow cytometry was performed on a BD FACSCalibur™ flow cytometry system. Positive selection of human B lymphocytes (bottom panels). Peripheral blood mononuclear cells (PBMC) were stained with APC Anti-Human CD19 (Cat. No. 555415) and FITC Anti-Human CD45RA (Cat. No. 555488), and then labeled with BD IMag™ Anti-APC Particles - DM. After labeling, the cells were separated using the BD IMag™ Cell Separation Magnet, and the negative (CD19-) and positive (CD19+) fractions were collected as described in the positive selection protocol. Please refer to the Positive Selection Flow Chart to identify the separated cell populations represented in this figure. Unseparated PBMC (left panel), the negative fraction (middle panel), and the positive fraction (right panel) were analyzed by flow cytometry. Nonviable cells were eliminated from analysis by staining with Propidium Iodide Staining Solution (Cat. No. 556463), and all viable leukocytes are displayed. The percentage of CD19+ B lymphocytes in each sample is given. Flow cytometry was performed on a BD FACSCalibur™ flow cytometry system.

Optimal concentrations of BD IMag™ Anti-APC Particles - DM for positive selection with some APC-conjugated monoclonal antibodies to human and mouse leukocyte antigens.


BD IMag™ APC Magnetic Particles - DM

BD IMag™ APC Magnetic Particles - DM

Regulatory Statusの凡例
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation and Storage
推奨アッセイ手順
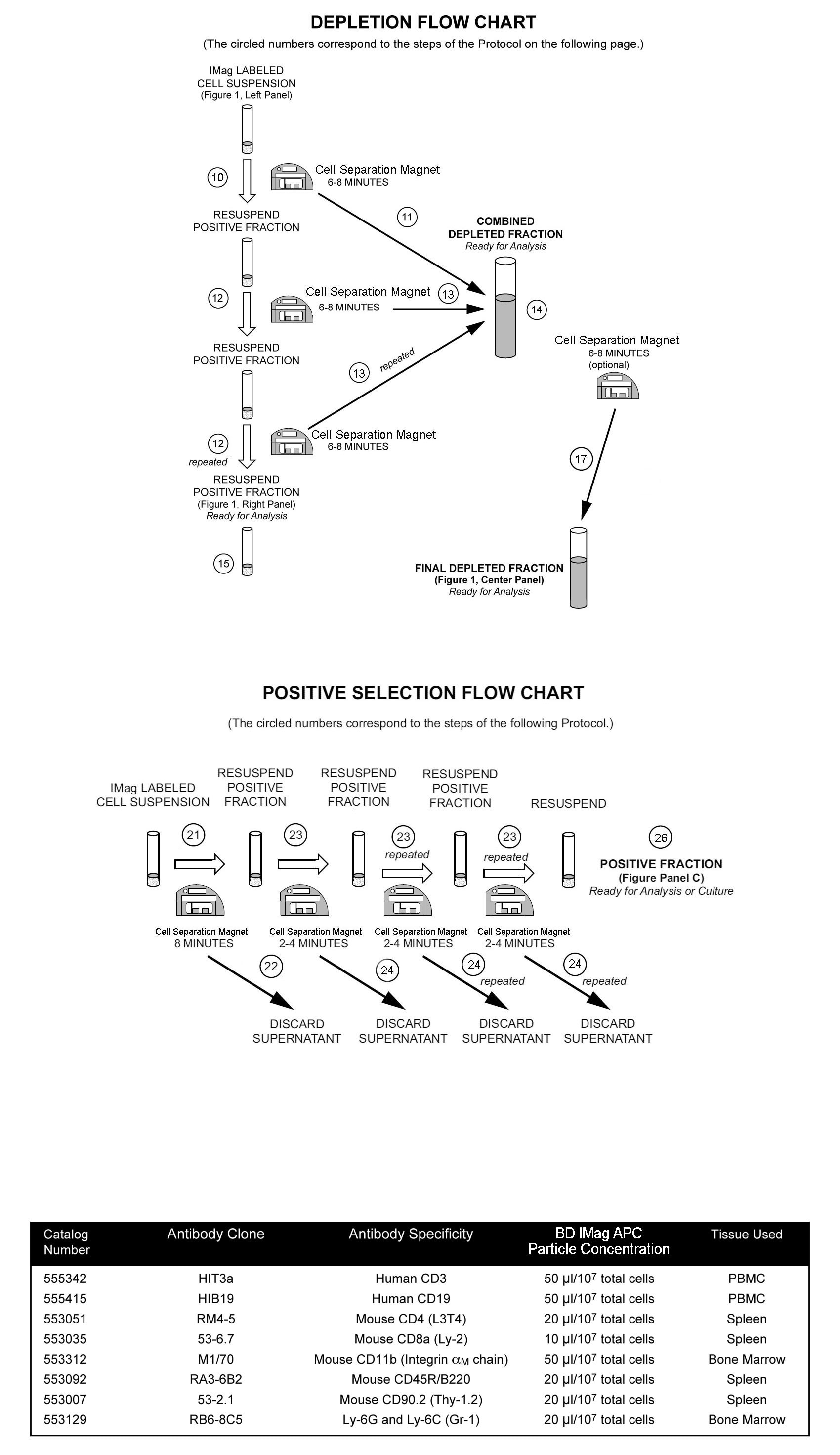
A detailed Magnetic Labeling and Separation Protocol follows. In brief, cells are labeled with the APC-conjugated antibody, which recognizes the subpopulation of interest. After washing away excess antibody, BD IMag™ Anti-APC Particles - DM are added to the cell suspension and bind the APC-conjugated antibody on the cells. The tube containing this labeled cell suspension is then placed within the magnetic field of the Cell Separation Magnet. Positive selection or depletion is then performed. Labeled cells migrate toward the magnet (positive fraction), leaving the unlabeled cells in suspension so they can be drawn off (depleted or negative fraction). The tube is then removed from the magnetic field for resuspension of the positive fraction. The selections are repeated twice to increase the purity of the positive fraction and the yield of the depleted fraction. The magnetic separation steps are diagrammed in the accompanying Depletion and Positive Selection Flow Charts. The small size of the BD IMag™ particles allows the positive fraction to be further evaluated in downstream applications such as flow cytometry.
MAGNETIC LABELING AND SEPARATION PROTOCOL
1. Prepare buffers and place on ice.
a. Cell-staining buffer: Phosphate Buffered Saline, 3% heat inactivated fetal calf serum, 0.1% sodium azide.
b. 1X BD IMag™ buffer: Dilute BD IMag™ Buffer (10X) (Cat. No. 552362) 1:10 with sterile distilled water or prepare Phosphate Buffered Saline, supplemented with 0.5% BSA, 2 mM EDTA, and 0.1% sodium azide.*
2. Aseptically prepare a single-cell suspension from the lymphoid tissue of interest or prepare PBMC from anti-coagulated blood, preferably by density gradient centrifugation using the appropriate density Ficoll-Paque solution. Remove clumps of cells and/or debris by passing the suspended cells through a 70-µm nylon cell strainer.
3. Count the cells, and resuspend them in cell-staining buffer at a concentration of 2 × 10e7 cells/ml.
4. Optional: If needed, add BD Mouse Fc Block™ Purified Anti-Mouse CD16/CD32 mAb 2.4G2 (Cat. No. 553141/553142) or BD Rat Fc Block™ Purified Anti-Rat CD32 mAb D34-485 (Cat. No. 550270/550271) at 0.25 µg/10e6 cells, and incubate on ice for 15 minutes.
5. Add the APC-conjugated antibody (or cocktail of APC-conjugated antibodies) at the appropriate concentration, and incubate on ice for 15 minutes. **
6. Wash the labeled cells with an excess volume of 1X BD IMag™ buffer, and carefully aspirate ALL the supernatant. For depletions, proceed with Step 7. For positive selections, proceed with Step 18.
Depletions:
7. Vortex the BD IMag™ Anti-APC Particles - DM thoroughly, and add 50 µl of particles for every 1 × 10e7 total cells.
8. MIX THOROUGHLY. Refrigerate for 30 minutes at 6°C - 12°C.
9. Bring the labeling volume up to a concentration of 2 to 8 × 10e7 cells/ml with 1X BD IMag™ buffer or culture medium.*
10. Transfer the labeled cells to a 12 × 75 mm round-bottom test tube, maximum volume added not to exceed 1.0 ml. Place this positive-fraction tube on the Cell Separation Magnet (horizontal position) for 6 to 8 minutes.
- For greater volume, transfer the cells to a 17 × 100 mm round-bottom test tube, maximum volume added not to exceed 3.0 ml. Place this positive-fraction tube on the Cell Separation Magnet (vertical position) for 8 minutes.
11. With the tube on the Cell Separation Magnet and using a glass Pasteur pipette, carefully aspirate the supernatant (depleted fraction) and place in a new tube.
12. Remove the positive-fraction tube from the Cell Separation Magnet, and add 1X BD IMag™ buffer (or medium) to the same volume as in Step 9. Resuspend the positive fraction well by pipetting up and down 10 to 15 times and place back on the Cell Separation Magnet for 6 to 8 minutes.
- 17 × 100 mm tube: Place on the Cell Separation Magnet for 8 minutes.
13. Using a new Pasteur pipette, carefully aspirate the supernatant and combine with the depleted fraction from Step 11 above.
14. Repeat Steps 12 and 13. The combined depleted fraction contains cells with no bound antibodies or magnetic particles. These cells are ready for downstream applications, or they can be further enriched by proceeding Step 16.
15. The positive-fraction cells remaining in the original tube can be resuspended in an appropriate buffer or culture medium for downstream applications, including flow cytometry.
16. To increase the purity of the combined depleted fraction, place the tube on the Cell Separation Magnet for another 6 to 8 minutes.
- 17 × 100 mm tube: Place on the Cell Separation Magnet for 8 minutes.
17. Carefully aspirate the supernatant and place in a new tube. This is the final depleted fraction. The cells are ready to be processed for downstream applications.
Positive Selections:
18. Vortex the BD IMag™ Anti-APC Particles - DM thoroughly, and add 10 to 50 µl of particles for every 1 × 10e7 total cells. The amount of particles to add will vary depending on how many cells one is targeting and the cell-surface density of the antigen. Please refer to the table below for some common examples.
19. MIX THOROUGHLY. Refrigerate mouse or rat leukocytes for 30 minutes at 6°C - 12°C. Incubate human PBMC at room temperature for 30 minutes.
20. Bring the labeling volume up to 2 to 8 × 10e7 cells/ml with 1X BD IMag™ buffer.
21. Immediately place the tube onto the Cell Separation Magnet and incubate for 6 to 8 minutes.
22. With the tube on the Cell Separation Magnet, carefully aspirate the supernatant. This supernatant is considered the Negative Fraction.
23. Remove the tube from the Cell Separation Magnet, and add 1X BD IMag™ buffer to the same volume as in Step 20. Gently resuspend the cells by pipetting up and down, and return the tube to the Cell Separation Magnet for another 2 to 4 minutes.
24. With the tube on the Cell Separation Magnet, carefully remove the supernatant.
25. Repeat Steps 23 and 24.
26. After the final wash step, remove the tube from the Cell Separation Magnet. Resuspend the positive fraction in an appropriate buffer or culture medium, and proceed with desired downstream application(s), including flow cytometry.
NOTES:
* For depletion of mouse leukocytes, tissue culture medium usually results in a slight increase in viability and recovery, when compared to IMag buffer, without reducing cell purity. We recommend that researchers run a trial comparison of media to buffer to make sure that there are no adverse effects.
** Avoid non-specific labeling by working quickly and adhering to recommended incubation times.
Product Notices
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- BD IMag™ particles are prepared from carboxy-functionalized magnetic particles which are manufactured by Skold Technology and are licensed under US patent number 7,169,618.
- Ficoll-Paque is a trademark of Amersham Biosciences Limited.
- This APC-conjugated reagent can be used in any flow cytometer equipped with a dye, HeNe, or red diode laser.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
関連製品
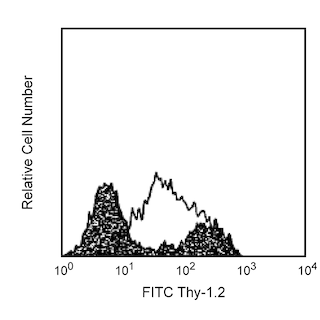


BD IMag™ Anti-Allophycocyanin (APC) Particles - DM are magnetic nanoparticles that have monoclonal antibodies conjugated to their surfaces. These particles are optimized for the positive selection or depletion of leukocyte subpopulations using the BD IMag™ Cell Separation Magnet (Cat. No. 552311). The E30-221 antibody reacts with APC, a commonly used fluorochrome for flow cytometry. The binding of the E30-221 antibody to APC does not quench the fluorescence of the APC molecule.
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.