-
抗体試薬
- フローサイトメトリー用試薬
-
ウェスタンブロッティング抗体試薬
- イムノアッセイ試薬
-
シングルセル試薬
- BD® AbSeq Assay | シングルセル試薬
- BD Rhapsody™ Accessory Kits | シングルセル試薬
- BD® Single-Cell Multiplexing Kit | シングルセル試薬
- BD Rhapsody™ Targeted mRNA Kits | シングルセル試薬
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit | シングルセル試薬
- BD Rhapsody™ TCR/BCR Profiling Assays (VDJ Assays) | シングルセル試薬
- BD® OMICS-Guard Sample Preservation Buffer
-
細胞機能評価のための試薬
-
顕微鏡・イメージング用試薬
-
細胞調製・分離試薬
-
- BD® AbSeq Assay | シングルセル試薬
- BD Rhapsody™ Accessory Kits | シングルセル試薬
- BD® Single-Cell Multiplexing Kit | シングルセル試薬
- BD Rhapsody™ Targeted mRNA Kits | シングルセル試薬
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit | シングルセル試薬
- BD Rhapsody™ TCR/BCR Profiling Assays (VDJ Assays) | シングルセル試薬
- BD® OMICS-Guard Sample Preservation Buffer
- Japan (Japanese)
-
Change country/language
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD FACS™ Pre-Sort Buffer
(RUO)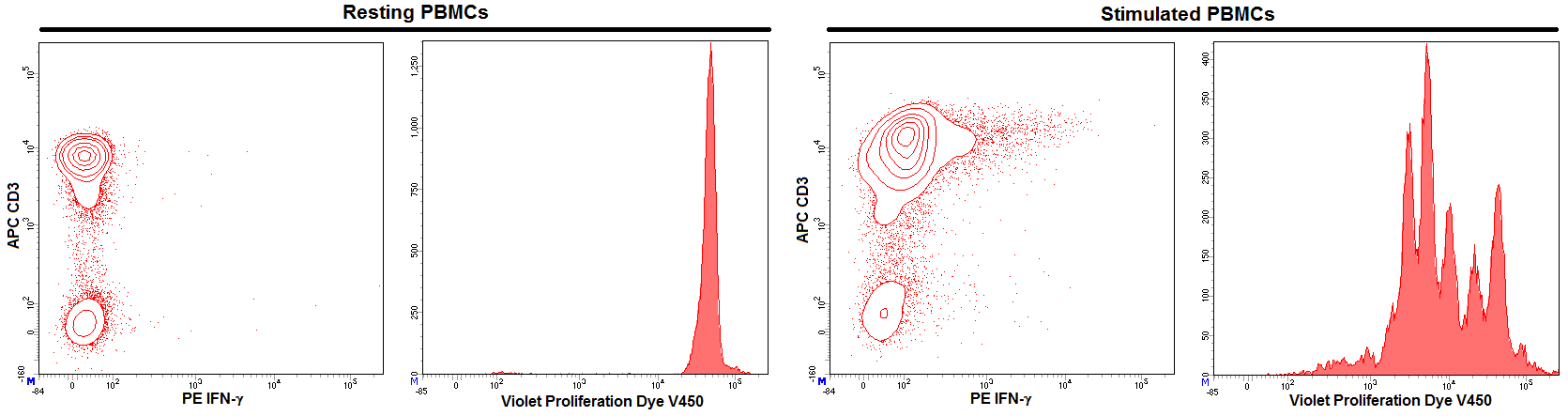




Activation of Sorted Lymphocytes. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient separation (Ficoll-Paque™, GE Healthcare). PBMCs were then washed and resuspended in Pre-Sort Buffer prior to cell sorting and maintained in Pre-Sort Buffer during sort on a BD FACSAria™ III Flow Cytometer system. Lymphocytes were sorted based on light scatter properties and were allowed to recover in culture for 24 hours in growth medium. At the end of the recovery period, cells were stained with BD Horizon™ Violet Proliferation Dye 450 (VPD, Cat. No. 562158) and stimulated with Dynabeads® Human T-Activator CD3/CD28, as per manufacturer's instructions (Life Technologies). After three days of stimulation, cells were treated with the protein transporter inhibitor BD GolgiStop™ (Cat. No. 554724) for 6 hours, then fixed and permeabilized with BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit (Cat. No. 554714), and stained with APC Mouse Anti-Human CD3 (Cat No. 555335) and PE Mouse Anti-Human IFN-γ (Cat. No. 559326). Flow cytometry was performed on a BD LSRFortessa™ flow cytometry system. Sorted cells not activated with CD3/CD28 beads were used as a negative control. As expected, no expression of IFN-γ or proliferation was observed within the lymphocyte population. After three day exposure to CD3/CD28 beads, stimulated CD3+ lymphocytes expressed IFN-γ and proliferated, as shown by the serial decrease in VPD intensity.

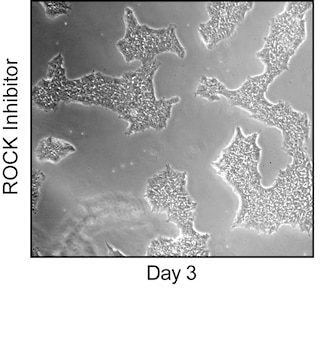
Post sort analysis of human embryonic stem cells (hESCs). H9 hESCs (WiCell, Madison WI) were dissociated using BD™ Accutase™ Cell Detachment Solution (Cat. No. 561527). Cells were washed, stained with Alexa Fluor® 647 Mouse anti-Human TRA-1-81 (Cat. No. 560793) and BD Horizon™ BV421 Rat anti-SSEA-3 (Cat No. 562706), and maintained in Pre-Sort Buffer during sort . Cells were sorted on a BD FACSAria™ III Flow Cytometer system. The sorted cells were then plated in 6 well tissue culture dishes at approximately 2 million cells per well with BD™ ROCK Inhibitor (Y-27632) (Cat. No. 562822) for 24 hours and cultured for 4 days. Cells were then analyzed for pluripotency. The cultures were dissociated using BD Accutase Cell Detachment Solution, fixed with BD Cytofix™ Fixation Buffer (Cat. No. 554655) and permeabilized with BD Phosflow™ Perm Buffer III (Cat. No. 558050). The cells were then stained with Alexa Fluor® 488 Mouse anti-Oct3/4 (Human Isoform A) (Cat. No. 561628), PE Mouse anti-Human Nanog (Cat. No. 560483), and Alexa Fluor® 647 Mouse Anti-Sox2 (Cat. No. 562139). Cells were then washed and analyzed using a BD LSRFortessa™ X-20 cytometry system. Sorted cells retained a pluripotent phenotype post-sort as shown by the expression of Oct4, Sox2, and Nanog.


BD Pharmingen™ Pre-Sort Buffer

BD Pharmingen™ Pre-Sort Buffer

Pre-Sort Buffer
Regulatory Statusの凡例
Becton, Dickinson and Companyの書面による明示的な許諾を得た使用以外での製品の使用は固く禁じられています。
説明
BD FACS™ Pre-Sort Buffer was designed to aid in preparing and maintaining single cell suspensions prepared from lymphoid tissues, bone marrow, peripheral blood, cultured cells (including pluripotent stem cells) or other sources. Additives may be necessary for sensitive cell types requiring specific nutrients. The Pre-Sort Buffer contains no phenol red and is an optically clear buffer to help minimize background. The buffer has been formulated to minimize cell clumping which facilitates consistent drop formation on fluorescence activated cell sorters. The buffer has minimal calcium and magnesium to minimize cell aggregation as these cations are necessary cofactors for many cell adhesion molecules. The Pre-Sort Buffer contains an FBS-based protein to help maintain cells in a viable state during cell sorting applications but the buffer does not contain EDTA, which can have deleterious effects on cells. It is useful for the dilution and application of fluorescent reagents as well as for the suspension, washing, and storage of cells destined for florescence activated cell sorting (FACS) or flow cytometric analysis.
調製と保管タイトルテキスト
Thaw completely at 4°C before first use. Distribute 25 ml aliquots (or desired single use volumes) of complete buffer into sterile containers and store at -20°C. The complete buffer is stable at -20°C for 6 months. Each aliquot should be considered single use and used completely on the day of thaw to ensure optimal activity (avoid multiple freeze/thaw cycles).
推奨アッセイ手順
Flow Cytometry (Direct immunofluorescence staining):
1. Thaw your BD FACS™ Pre-Sort Buffer aliquot completely at 4°C.
2. Prepare single-cell suspensions from tissue, bone marrow, peripheral blood or cell cultures using standard protocols.
3. Wash the cells twice in cold complete Pre-Sort Buffer. Resuspend the cell pellet with cold Pre-Sort Buffer to a final concentration of ~1 x 10e7 cells/ml.
a. If necessary dilute fluorescent antibodies to their predetermined optimal concentrations in complete Pre-Sort Buffer.
4. Add appropriate amount of conjugated antibody to the cell suspension. Incubate for 20 minutes on ice protected from light.
a. Staining time may be increased (≥ 45 min) depending on the avidity of the fluorescently conjugated antibody.
5. Wash the cells two times with complete Pre-Sort Buffer. Centrifuge cells at 300 x g for 5 min. After each centrifugation, carefully aspirate or invert and blot away supernatants from cell pellets.
6. Resuspend the cell pellet in complete Pre-Sort Buffer and transfer stained cells to the appropriate tubes for flow cytometric analysis and/or sorting (e.g. adjust final volume to provide ~5 x 10e6 cells/ml).
7. Immediately sort cells based on an optimized sort protocol.
Note 1: DNase II can be added to Pre-Sort Buffer to help discourage clumping of cell suspensions that have a high amount of cell death. The presence of free DNA in cell suspension can contribute to cell clumping. DNase I is not compatible with the Pre-Sort Buffer due to the necessity of cations for DNase I to function. DNase II contains levels of endotoxin and may vary by batch. Depending on your application, you may wish to add DNase II to the Pre-Sort Buffer. However, you may also need to determine if the levels of endotoxin are appropriate for your application.
Note 2: Pre-Sort Buffer does not contain antibiotics. Antibiotics can be added to the Pre-Sort Buffer as appropriate.
Note 3: Pre-Sort Buffer can similarly be used for the indirect immunofluorescent staining of cells. In this case, repeat steps 4 and 5 with second step reagents when using either unlabeled or biotinylated primary antibodies.
Note 4: Pre-Sort Buffer can also be used for the immunofluorescent staining of surface antigens expressed by cells that are destined to be fixed and immunofluorescently stained for intracellular antigens such as cytokines and transcription factors. Cells stained for intracellular antigens can be resuspended and maintained (i.e., at 4°C, protected from light) in Pre-Sort Buffer prior to analysis by flow cytometry.
Note 5: Pre-Sort Buffer is not a post-sort collection buffer. Please collect cells in buffer/media that is appropriate for downstream applications.
Note 6: Pre-Sort Buffer is compatible with commonly used viability dyes such as 7-AAD.
製品通知
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- Accutase is a registered trademark of Innovative Cell Technologies, Inc.
- Ficoll-Paque is a trademark of Amersham Biosciences Limited.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
コンパニオン製品

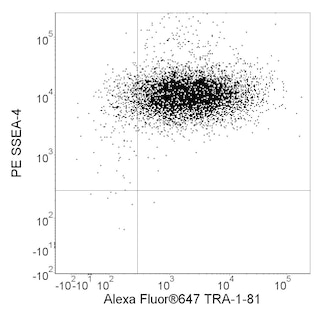
Development References (1)
-
McIntyre CA, Renin G. Reduction in Endotoxin Levels After Performing the Prepare for Aseptic Sort Procedure on the BD FACSAria II Flow Cytometer. Available: http://www.bdbiosciences.com/documents/FacsariaII_Endotoxin.pdf 2015, February 9.
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.