-
抗体試薬
- フローサイトメトリー用試薬
-
ウェスタンブロッティング抗体試薬
- イムノアッセイ試薬
-
シングルセル試薬
- BD® AbSeq Assay | シングルセル試薬
- BD Rhapsody™ Accessory Kits | シングルセル試薬
- BD® Single-Cell Multiplexing Kit | シングルセル試薬
- BD Rhapsody™ Targeted mRNA Kits | シングルセル試薬
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit | シングルセル試薬
- BD Rhapsody™ TCR/BCR Profiling Assays (VDJ Assays) | シングルセル試薬
- BD® OMICS-Guard Sample Preservation Buffer
-
細胞機能評価のための試薬
-
顕微鏡・イメージング用試薬
-
細胞調製・分離試薬
-
- BD® AbSeq Assay | シングルセル試薬
- BD Rhapsody™ Accessory Kits | シングルセル試薬
- BD® Single-Cell Multiplexing Kit | シングルセル試薬
- BD Rhapsody™ Targeted mRNA Kits | シングルセル試薬
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit | シングルセル試薬
- BD Rhapsody™ TCR/BCR Profiling Assays (VDJ Assays) | シングルセル試薬
- BD® OMICS-Guard Sample Preservation Buffer
- Japan (Japanese)
-
Change country/language
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?




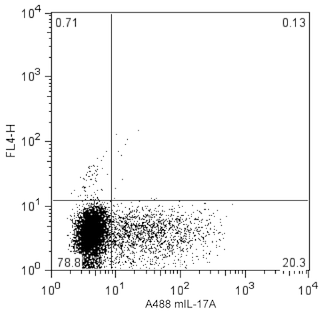
Flow cytometric analysis of mouse IL-17F expression in resting or stimulated mouse EL4 thymoma cells or in polarized mouse Th17 cells. Mouse EL4 thymoma cells were either unstimulated or stimulated with Phorbol 12-Myristate 13-Acetate (PMA; Sigma P-8139) and Ionomycin (Sigma; I-0634) in the presence of BD GolgiStop™ Protein Transport Inhibitor (Cat. No. 554724) for 5 hours. Th17 cells were generated from Th17-polarizing mouse spleen cell cultures and were stimulated with PMA and Ionomycin with BD GolgiStop™ Protein Transport Inhibitor. The EL4 and Th17 cells were fixed and permeabilized using the BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit (Cat. No. 554714). The unstimulated (Left Panel) and stimulated (Middle Panel) EL4 cells were stained with PerCP-Cy™5.5 Mouse Anti-Mouse IL-17F (Cat. No. 562194). To validate O79-289 antibody use for staining IL-17F in Th17 cells generated from primary mouse T cells, the fixed and permeabilized Th17-polarized cells were stained with purified O79-289 Mouse Anti-Mouse IL-17F antibody followed by PE Goat Anti-Mouse Ig (Cat. No. 550589) and with Alexa Fluor® 488 Rat Anti-Mouse IL-17A antibody (Cat. No. 560220) (Right Panel). Two-color flow cytometric dot plots showing the correlated expression patterns of IL-17F versus cellular autofluorescence (measured in the FL1 channel) for EL4 cells or versus IL-17A for Th17 cells were derived from gated events with the forward and side light-scatter characteristics of intact cells. Flow cytometry was performed using a BD™ LSR II Flow Cytometry System.


BD Pharmingen™ PerCP-Cy™5.5 Mouse Anti-Mouse IL-17F

Regulatory Statusの凡例
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation and Storage
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Cy is a trademark of Amersham Biosciences Limited. This conjugated product is sold under license to the following patents: US Patent Nos. 5,486,616; 5,569,587; 5,569,766; 5,627,027.
- Please observe the following precautions: Absorption of visible light can significantly alter the energy transfer occurring in any tandem fluorochrome conjugate; therefore, we recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to prevent exposure of conjugated reagents, including cells stained with those reagents, to room illumination.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- PerCP-Cy5.5–labelled antibodies can be used with FITC- and R-PE–labelled reagents in single-laser flow cytometers with no significant spectral overlap of PerCP-Cy5.5, FITC, and R-PE fluorescence.
- PerCP-Cy5.5 is optimized for use with a single argon ion laser emitting 488-nm light. Because of the broad absorption spectrum of the tandem fluorochrome, extra care must be taken when using dual-laser cytometers, which may directly excite both PerCP and Cy5.5™. We recommend the use of cross-beam compensation during data acquisition or software compensation during data analysis.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- This product is subject to proprietary rights of Amersham Biosciences Corp. and Carnegie Mellon University and made and sold under license from Amersham Biosciences Corp. This product is licensed for sale only for research. It is not licensed for any other use. If you require a commercial license to use this product and do not have one return this material, unopened to BD Biosciences, 10975 Torreyana Rd, San Diego, CA 92121 and any money paid for the material will be refunded.
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
関連製品


.png?imwidth=320)
The O79-289 monoclonal antibody specifically binds to the inflammatory cytokine protein, Interleukin-17 (IL-17F). IL-17F is a member of the IL-17 family of cytokines. Among IL-17 family members, IL-17F has the highest amino acid sequence homology to IL-17A. IL-17F is produced by activated CD4+ T helper (Th17) cells, CD8+ T (Tc17) cells and γδ T cells. IL-17F can be secreted as homodimers or as heterodimers with IL-17A. IL-17F and IL-17A have overlapping functions such as inducing epithelial cells and fibroblasts to produce proinflammatory cytokines and chemokines including IL-6, GM-CSF, CXCL1, CCL2, and CCL7. These factors attract and activate neutrophils and other cell types that mediate protective responses against pathogenic microbes or pathologic allergic or autoimmune diseases. IL-17 gene knockout studies have shown that IL-17F and IL-17A have independent functions as well. IL-17F and IL-17A exert their biological function by binding to and signaling through IL-17 receptors comprised of the transmembrane receptor subunits, IL-17RA (CD217) and IL-17RC.
Development References (8)
-
Chang SH, Dong C. IL-17F: Regulation, signaling and function in inflammation. Cytokine. 2009; 46(1):7-11. (Biology). View Reference
-
Dong C. Th17 cells: Current understanding of their generation and regulation. Eur J Immunol. 2009; 39(3):640-644. (Biology). View Reference
-
Hamada H, Garcia-Hernandez MdlL, Reome JB, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009; 182(6):3469-3481. (Biology). View Reference
-
Ishigame H, Kakuta S, Nagai T, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009; 30(1):108-119. (Biology). View Reference
-
Martinez GJ, Zhang Z, Chung Y, et al. Smad3 differentially regulates the induction of regulatory and inflammatory T cell differentiation. J Biol Chem. 2009; 284(51):35283-35286. (Biology). View Reference
-
Oda N, Canelos PB, Essayan DM, Plunkett BA, Myers AC, Huang SK. Interleukin-17F induces pulmonary neutrophilia and amplifies antigen-induced allergic response. Am J Respir Crit Care Med. 2005; 171(1):12-13. (Biology). View Reference
-
Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009; 31(2):331-341. (Biology). View Reference
-
Yang XO, Chang SH, Park H, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008; 205(5):1063-1075. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.