-
Your selected country is
Middle East / Africa
- Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
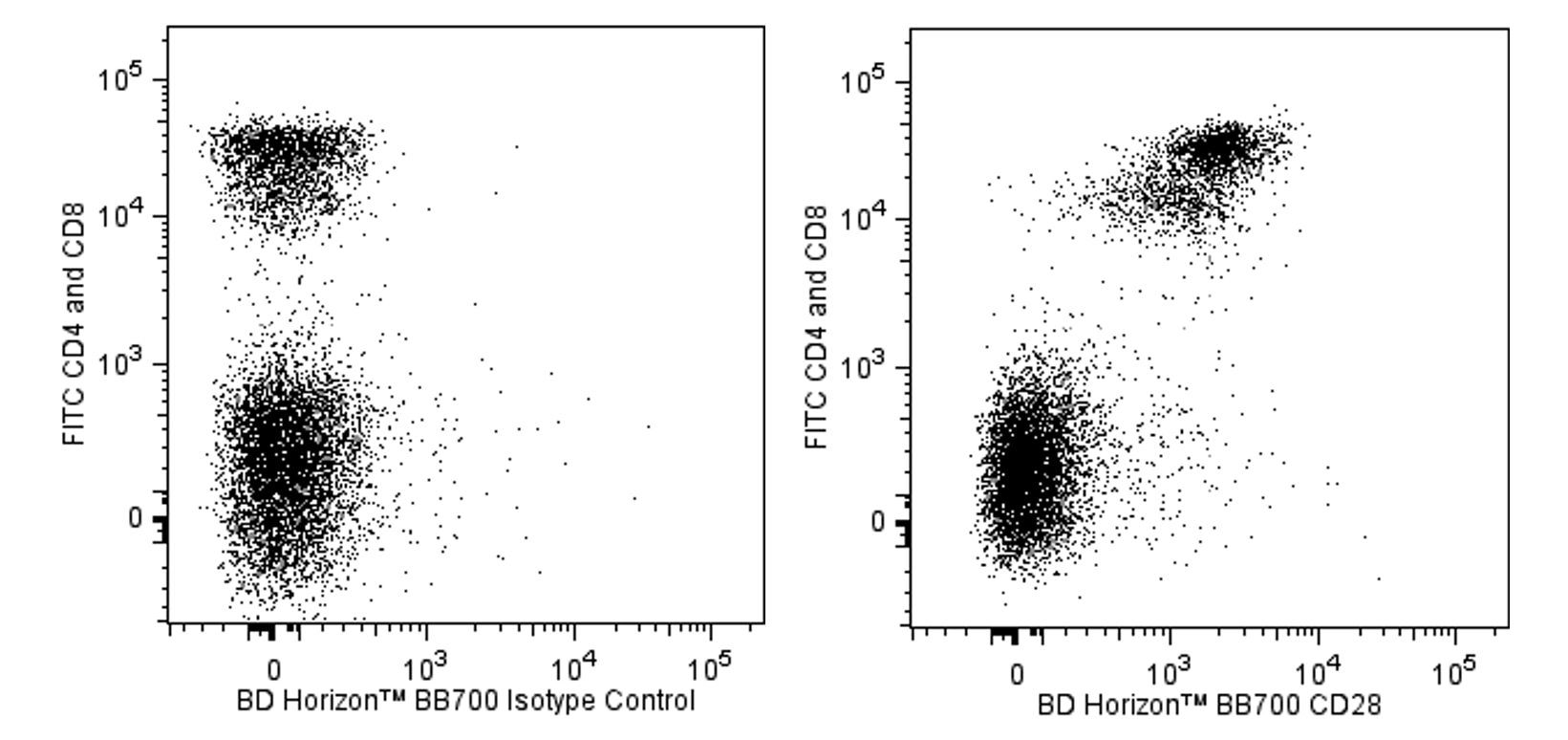



Multicolor flow cytometric analysis of CD28 expression on C57BL/6 mouse splenocytes. Splenic leucocytes were pre-incubated with Purified Rat Anti-Mouse CD16/CD32 antibody (Mouse BD Fc Block™) (Cat. No. 553141/553142). The cells were then stained simultaneously with FITC Rat Anti-Mouse CD4 (Cat. No. 553046/553047/561835) and FITC Rat Anti-Mouse CD8 (Cat. No. 553030/553031/561966) antibodies and with either BD Horizon™ BB700 Hamster IgG2, κ Isotype Control (Cat. No. 566422; Left Plot) or BD Horizon BB700 Hamster Anti-Mouse CD28 antibody (Cat. No. 566512/566513; Right Plot) at 0.5 µg/test. BD Pharmingen™ DAPI Solution (Cat. No. 564907) was added to cells right before analysis. Two-color flow cytometric dot plots showing the correlated expression patterns of CD28 (or Ig Isotype Control staining) versus CD4 and CD8 were derived for DAPI-negative gated events with the forward and side light-scatter characteristics of viable leucocytes. Flow cytometric analysis was performed using a BD FACSCelesta™ Flow Cytometer System.


BD Horizon™ BB700 Hamster Anti-Mouse CD28

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
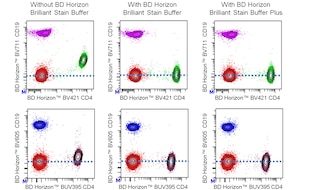
For optimal and reproducible results, BD Horizon Brilliant Stain Buffer should be used anytime two or more BD Horizon Brilliant dyes are used in the same experiment. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. More information can be found in the Technical Data Sheet for the BD Horizon Brilliant Stain Buffer (Cat. No. 563794/566349) or the BD Horizon Brilliant Stain Buffer Plus (Cat. No. 566385).
When setting up compensation, it is recommended to compare spillover values obtained from cells and BD™ CompBeads to ensure that beads will provide sufficiently accurate spillover values.
For optimal results, it is recommended to perform two washes after staining with antibodies. Cells may be prepared, stained with antibodies and washed twice with wash buffer per established protocols for immunofluorescent staining, prior to acquisition on a flow cytometer. Performing fewer than the recommended wash steps may lead to increased spread of the negative population.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- The manufacture, use, sale, offer for sale, or import of this product is subject to one or more patents or pending applications. This product, and only in the amount purchased by buyer, may be used solely for buyer’s own internal research, in a manner consistent with the accompanying product literature. No other right to use, sell or otherwise transfer (a) this product, or (b) its components is hereby granted expressly, by implication or by estoppel. Diagnostic uses require a separate license.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- BD Horizon Brilliant Blue 700 is covered by one or more of the following US patents: 8,455,613 and 8,575,303.
- Cy is a trademark of GE Healthcare.
- Although hamster immunoglobulin isotypes have not been well defined, BD Biosciences Pharmingen has grouped Armenian and Syrian hamster IgG monoclonal antibodies according to their reactivity with a panel of mouse anti-hamster IgG mAbs. A table of the hamster IgG groups, Reactivity of Mouse Anti-Hamster Ig mAbs, may be viewed at http://www.bdbiosciences.com/documents/hamster_chart_11x17.pdf.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products




The 37.51 antibody reacts with CD28, which is expressed on most thymocytes, at low density on nearly all CD4+ and CD8+ peripheral T cells, and at even lower density on NK cells. The expression of CD28, in splenocytes and thymocytes, has been reported to increase after activation. CD28 transcripts are found in mast cells, and cell-surface expression of CD28 is induced upon maturation or activation of mast cells. It has been reported that CD28 is not expressed on some populations of intraepithelial T lymphocytes. CD28 is a costimulatory receptor; its ligands include CD80 (B7-1) and CD86 (B7-2). The 37.51 mAb augments proliferation and cytokine production by activated T and NK cells and can provide a costimulatory signal for CTL induction. There is considerable evidence that CD28 is a costimulatory receptor involved in many, but not all, T cell-dependent immune responses.
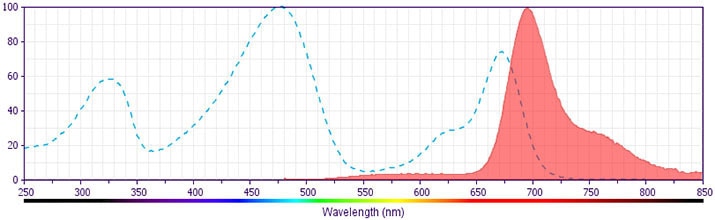
The antibody was conjugated to BD Horizon BB700, which is part of the BD Horizon Brilliant™ Blue family of dyes. It is a polymer-based tandem dye developed exclusively by BD Biosciences. With an excitation max of 485 nm and an emission max of 693 nm, BD Horizon BB700 can be excited by the 488 nm laser and detected in a standard PerCP-Cy™5.5 set (eg, 695/40-nm filter). This dye provides a much brighter alternative to PerCP-Cy5.5 with less cross laser excitation off the 405 nm and 355 nm lasers.
Development References (14)
-
Cibotti R, Punt JA, Dash KS, Sharrow SO, Singer A. Surface molecules that drive T cell development in vitro in the absence of thymic epithelium and in the absence of lineage-specific signals. Immunity. 1997; 6(3):245-255. (Clone-specific: (Co)-stimulation, Stimulation). View Reference
-
Gelfanov V, Lai YG, Gelfanova V, Dong JY, Su JP, Liao NS. Differential requirement of CD28 costimulation for activation of murine CD8+ intestinal intraepithelial lymphocyte subsets and lymph node cells. J Immunol. 1995; 155(1):76-82. (Clone-specific: (Co)-stimulation, Flow cytometry, Stimulation). View Reference
-
Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992; 149(2):380-388. (Immunogen: (Co)-stimulation, Flow cytometry, Immunoprecipitation, Stimulation). View Reference
-
Harding FA, Allison JP. CD28-B7 interactions allow the induction of CD8+ cytotoxic T lymphocytes in the absence of exogenous help. J Exp Med. 1993; 177(6):1791-1796. (Clone-specific: (Co)-stimulation, Inhibition, Stimulation). View Reference
-
Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992; 356(6370):607-609. (Clone-specific: (Co)-stimulation, Stimulation). View Reference
-
June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994; 15(7):321-331. (Biology). View Reference
-
Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995; 182(2):459-465. (Clone-specific: (Co)-stimulation, Stimulation). View Reference
-
Lepesant H, Pierres M, Naquet P. Deficient antigen presentation by thymic epithelial cells reveals differential induction of T cell clone effector functions by CD28-mediated costimulation. Cell Immunol. 1995; 161(2):279-287. (Clone-specific: (Co)-stimulation, Stimulation). View Reference
-
Marietta EV, Weis JJ, Weis JH. CD28 expression by mouse mast cells is modulated by lipopolysaccharide and outer surface protein A lipoprotein from Borrelia burgdorferi. J Immunol. 1997; 159(6):2840-2848. (Clone-specific: (Co)-stimulation, Flow cytometry, Stimulation). View Reference
-
Nandi D, Gross JA, Allison JP. CD28-mediated costimulation is necessary for optimal proliferation of murine NK cells. J Immunol. 1994; 152(7):3361-3369. (Clone-specific: (Co)-stimulation, Flow cytometry, Stimulation). View Reference
-
Ong CJ, Lim AS, Teh HS. CD28-induced cytokine production and proliferation by thymocytes are differentially regulated by the p59fyn tyrosine kinase. J Immunol. 1997; 159(5):2169-2176. (Clone-specific: (Co)-stimulation, Stimulation). View Reference
-
Rakasz E, Hagen M, Sandor M, Lynch RG. Gamma delta T cells of the murine vagina: T cell response in vivo in the absence of the expression of CD2 and CD28 molecules. Int Immunol. 1997; 9(1):161-167. (Clone-specific: Flow cytometry). View Reference
-
Shahinian A, Pfeffer K, Lee KP, et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993; 261(5121):609-612. (Biology). View Reference
-
Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Invest. 1997; 100(12):3173-3183. (Clone-specific: (Co)-stimulation, Stimulation). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.