-
Your selected country is
Middle East / Africa
- Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


.png)

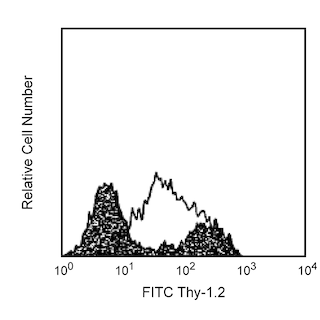
Flow cytometric analysis of CD86 on mouse splenocytes. Left Panel: LPS-stimulated C57BL/6 splenocytes (72 hr) were stained either with a PE-Cy™7 Rat IgG2a, κ isotype control (shaded) or with the PE-Cy™7 Rat Anti-Mouse CD86 antibody (unshaded). Right Panel: Freshly isolated unstimulated C57BL/6 splenocytes (shaded) and LPS-stimulated C57BL/6 splenocytes (72 hr) (unshaded) were stained with the PE-Cy™7 Rat Anti-Mouse CD86 antibody. Histograms were derived from gated events based on light scattering characteristics for lymphocytes. Flow cytometry was performed on a BD™ LSR II flow cytometry system.
.png)

BD Pharmingen™ PE-Cy™7 Rat Anti-Mouse CD86
.png)
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- Warning: Some APC-Cy7 and PE-Cy7 conjugates show changes in their emission spectrum with prolonged exposure to formaldehyde. If you are unable to analyze fixed samples within four hours, we recommend that you use BD™ Stabilizing Fixative (Cat. No. 338036).
- Cy is a trademark of Amersham Biosciences Limited. This conjugated product is sold under license to the following patents: US Patent Nos. 5,486,616; 5,569,587; 5,569,766; 5,627,027.
- This product is subject to proprietary rights of Amersham Biosciences Corp. and Carnegie Mellon University and made and sold under license from Amersham Biosciences Corp. This product is licensed for sale only for research. It is not licensed for any other use. If you require a commercial license to use this product and do not have one return this material, unopened to BD Biosciences, 10975 Torreyana Rd, San Diego, CA 92121 and any money paid for the material will be refunded.
- PE-Cy7 is a tandem fluorochrome composed of R-phycoerythrin (PE), which is excited by 488-nm light and serves as an energy donor, coupled to the cyanine dye Cy7, which acts as an energy acceptor and fluoresces maximally at 780 nm. PE-Cy7 tandem fluorochrome emission is collected in a detector for fluorescence wavelengths of 750 nm and higher. Although every effort is made to minimize the lot-to-lot variation in the efficiency of the fluorochrome energy transfer, differences in the residual emission from PE may be observed. Therefore, we recommend that individual compensation controls be performed for every PE-Cy7 conjugate. PE-Cy7 is optimized for use with a single argon ion laser emitting 488-nm light, and there is no significant overlap between PE-Cy7 and FITC emission spectra. When using dual-laser cytometers, which may directly excite both PE and Cy7, we recommend the use of cross-beam compensation during data acquisition or software compensation during data analysis.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Please observe the following precautions: Absorption of visible light can significantly alter the energy transfer occurring in any tandem fluorochrome conjugate; therefore, we recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to prevent exposure of conjugated reagents, including cells stained with those reagents, to room illumination.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products
.png?imwidth=320)
The GL1 antibody specifically recognizes the B7-2 (CD86) costimulatory molecule expressed on a broad spectrum of leukocytes, including B lymphocytes, T lymphocytes, thioglycollate-induced peritoneal macrophages, dendritic cells and astrocytes. CD86 is expressed at low levels by freshly explanted peripheral B and T cells, and its expression is substantially increased by a variety of T cell- and B cell-specific stimuli with a peak expression after 18-42 hours of culture. In contrast to most naive CD4+ T cells, memory CD4+ T cells express B7-2, both at the mRNA and protein level. CD86, a ligand for CD28 and CD152 (CTLA-4), is one of the accessory molecules that plays an important role in T cell-B cell costimulatory interactions. It has been shown to be involved in immunoglobulin class-switching and triggering of mouse NK cell-mediated cytotoxicity. CD80 (B7-1) is an alternate ligand for CD28 and CD152 (CTLA-4). GL1 antibody reportedly blocks MLR and stimulation of T cells by natural antigen-presenting cells. In addition, a mixture of anti-B7-1 and anti B7-2 (GL1) mAbs reportedly inhibits the in vitro interaction of CTLA-4 with its ligand and the in vivo priming of cytotoxic T lymphocytes.
Development References (20)
-
Bluestone JA. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995; 2(6):555-559. (Biology). View Reference
-
Borriello F, Sethna MP, Boyd SD, et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997; 6(3):303-313. (Biology). View Reference
-
Boussiotis VA, Gribben JG, Freeman GJ, Nadler LM. Blockade of the CD28 co-stimulatory pathway: a means to induce tolerance. Curr Opin Immunol. 1994; 6(5):797-807. (Biology). View Reference
-
Freeman GJ, Borriello F, Hodes RJ, et al. Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science. 1993; 262(5135):907-909. (Biology). View Reference
-
Hakamada-Taguchi R, Kato T, Ushijima H, Murakami M, Uede T, Nariuchi H. Expression and co-stimulatory function of B7-2 on murine CD4+ T cells. Eur J Immunol. 1998; 28(3):865-873. (Biology). View Reference
-
Hathcock KS, Laszlo G, Dickler HB, Bradshaw J, Linsley P, Hodes RJ. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993; 262(5135):905-907. (Immunogen). View Reference
-
Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994; 180(2):631-640. (Biology). View Reference
-
Herold KC, Vezys V, Koons A, Lenschow D, Thompson C, Bluestone JA. CD28/B7 costimulation regulates autoimmune diabetes induced with multiple low doses of streptozotocin. J Immunol. 1997; 158(2):984-991. (Biology). View Reference
-
Inaba K, Witmer-Pack M, Inaba M, et al. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994; 180(5):1849-1860. (Biology). View Reference
-
Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997; 278(5343):1626-1629. (Biology). View Reference
-
Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995; 182(2):459-465. (Biology). View Reference
-
Larsen CP, Ritchie SC, Hendrix R, et al. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J Immunol. 1994; 152(11):5208-5219. (Biology). View Reference
-
Lenschow DJ, Su GH, Zuckerman LA, et al. Expression and functional significance of an additional ligand for CTLA-4. Proc Natl Acad Sci U S A. 1993; 90(23):11054-11058. (Biology). View Reference
-
Martin-Fontecha A, Assarsson E, Carbone E, Karre K, Ljunggren HG. Triggering of murine NK cells by CD40 and CD86 (B7-2). J Immunol. 1999; 162(10):5910-5916. (Biology). View Reference
-
McAdam AJ, Schweitzer AN, Sharpe AH. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol Rev. 1998; 165:231-247. (Biology). View Reference
-
Nikcevich KM, Gordon KB, Tan L, et al. IFN-gamma-activated primary murine astrocytes express B7 costimulatory molecules and prime naive antigen-specific T cells. J Immunol. 1997; 158(2):614-621. (Biology). View Reference
-
Nuriya S, Yagita H, Okumura K, Azuma M. The differential role of CD86 and CD80 co-stimulatory molecules in the induction and the effector phases of contact hypersensitivity. Int Immunol. 1996; 8(6):917-926. (Biology). View Reference
-
Rauschmayr-Kopp T, Williams IR, Borriello F, Sharpe AH, Kupper TS. Distinct roles for B7 costimulation in contact hypersensitivity and humoral immune responses to epicutaneous antigen. Eur J Immunol. 1998; 28(12):4221-4227. (Biology). View Reference
-
Roy M, Aruffo A, Ledbetter J, Linsley P, Kehry M, Noelle R. Studies on the interdependence of gp39 and B7 expression and function during antigen-specific immune responses. Eur J Immunol. 1995; 25(2):596-603. (Biology). View Reference
-
Yang G, Mizuno MT, Hellstrom KE, Chen L. B7-negative versus B7-positive P815 tumor: differential requirements for priming of an antitumor immune response in lymph nodes. J Immunol. 1997; 158(2):851-858. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.