-
Your selected country is
Middle East / Africa
- Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Recombinant Human IL-2
(RUO)

Recombinant Human IL-2
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
Interleukin-2 (IL-2), originally called T cell growth factor (TCGF), is a multifunctional cytokine which can stimulate the differentiation and proliferation of T lymphocytes and other cell types including B lymphocytes, NK cells, LAK cells, and monocytes/macrophages. IL-2 exerts its biological effects by binding to specific receptors expressed by various target cells. Human IL-2 is a 15 kD protein containing 133 amino acid residues. Recombinant human IL-2 (Cat. No. 554603) is supplied as a frozen liquid comprised of 0.22 µm sterile-filtered aqueous buffered solution containing glycerol and bovine serum albumin, with no preservatives. Recombinant human IL-2 is ≥ 95% pure as determined by SDS-PAGE, and an absorbance assay based on the Beers-Lambert law. The endotoxin level is ≤ 0.1 ng per µg of human IL-2, as measured in a chromogenic LAL assay.
Preparation And Storage
Recommended Assay Procedures
Upon initial thawing, recombinant human IL-2 (Cat. No. 554603) should be aliquoted into polypropylene microtubes and frozen at -80°C for future use. Alternatively, the product can be diluted in sterile neutral buffer containing not less than 0.5 - 10 mg/mL carrier protein, such as human or bovine serum albumin, aliquoted and stored at -80°C. For in vitro biological assay use, carrier-protein concentrations of ≥ 1 mg/mL are recommended. For use as an ELISA standard, carrier-protein concentrations of 5 - 10 mg/mL are recommended. Failure to add carrier protein or store at indicated temperatures may result in a loss of activity. Recombinant human IL-2 should not be diluted to less than 10 µg/mL for long term storage. Carrier proteins should be pre-screened for possible effects in each investigator's experimental system. Carrier proteins may have an undesired influence on experimental results due to toxicity, high endotoxin levels or possible blocking activity.
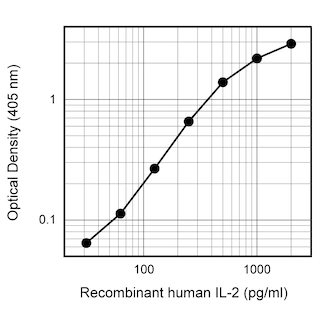
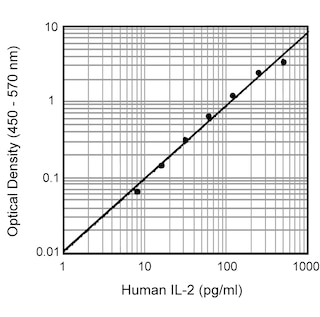
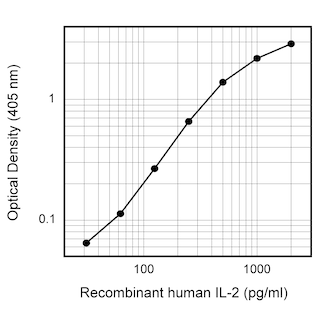
ELISA Standard: Recombinant human IL-2 (Cat. No. 554603) is useful as a quantitative standard for measuring human IL-2 protein levels using sandwich ELISA with purified 5344.111 (Cat. No. 555051) as a capture antibody and biotinylated B33-2 (Cat. No. 555040) as the detection antibody. To obtain linear standard curves, investigators may want to consider using doubling dilutions of recombinant human IL-2 from
2,000 - 15 pg/mL to be included in each ELISA plate. For measuring human IL-2 in serum or plasma, investigators are highly encouraged to use the BD OptEIA™ Human IL-2 Set (Cat. No. 555190) and BD OptEIA™ Human IL-2 Kit II (Cat. No. 550611 ).
Bioassay: Investigators are advised that the Bioassay application is not routinely tested for this material and are highly encouraged to both titrate this material and include appropriate controls in relevant experiments. An activity range of 0.06 - 1.0 x 10^9 units/mg, encompassing an
ED50 = 10 - 150 pg/mL, has previously been reported using CTLL-2 as indicator cells for proliferation, with a unit defined as the amount of material needed to stimulate a half-maximal response at cytokine saturation.
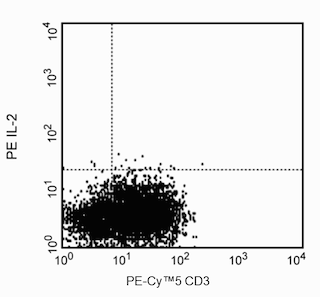
Blocking: Recombinant human IL-2 (Cat. No. 554603) can be useful as a blocking control for flow cytometric analysis when used with a fluorochrome-conjugated antibody, such as PE-conjugated MQ1-17H12 (Cat. No. 559334). Investigators are advised that the blocking application is not routinely tested for this material. Intracellular cytokine staining techniques and the use of blocking controls are described in detail by C.Prussin and D.Metcalfe.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- This product is manufactured and sold under license from Pestka Biomedical Laboratories, Inc. (d/b/a PBL InterferonSource) and may be used solely as indicated. This product may not be resold or incorporated in any manner into another product for resale. Any use for therapeutics is strictly prohibited. This product is covered by U.S. Patent No. 5,597,901 and Bulgarian Patent No. BG1895.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products
Development References (5)
-
Gillis S, Ferm MM, Ou W, Smith KA. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978; 120(6):2027-2032. (Biology). View Reference
-
Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995; 188(1):117-128. (Methodology). View Reference
-
Smith, KA. Interleukin-2: inception, impact, and implications. Science. 1988; 240(4856):1169-1176. (Biology). View Reference
-
Stern AS, Pan YC, Urdal DL, et al. Purification to homogeneity and partial characterization of interleukin 2 from a human T-cell leukemia. Proc Natl Acad Sci U S A. 1984; 81(3):871-875. (Biology). View Reference
-
Taniguchi T, Matsui H, Fujita T, et al. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983; 302(5906):305-310. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.