-
Your selected country is
Middle East / Africa
- Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Violet Live Cell Caspase Probe
(RUO)


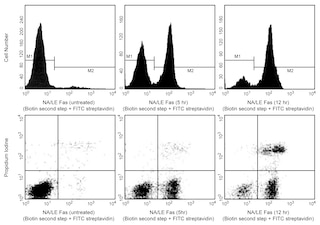
Two-color flow cytometric analysis of Viability and Caspase Activity in Camptothecin-treated Jurkat Cells. Cells from the human Jurkat (Acute T cell leukemia, ATCC TIB-152) cell line were treated with 0.025% DMSO (Left Panel) or 5 μM Camptothecin (Right Panel) for 16 hours. The cultured cells were then stained with BD Pharmingen™ Violet Live Cell Caspase Probe (Cat. No. 565521) according to the recommended assay procedure. After recovery in media, cells were resuspended in DPBS and stained with BD Horizon™ Fixable Viability Stain 780 (FVS780 , Cat. No. 565388). Two-color flow cytometric contour plots showing the correlated expression of Violet Live Caspase Probe fluorescence versus BD Horizon™ Fixable Viability Stain 780 fluorescence were derived from gated events with the forward and side light-scatter characteristics of viable cells. Live cells are double negative for both fluorescent probes, whereas apoptotic cells are positive for Violet Live Cell Caspase Probe and negative for FVS780, and dead cells are positive for FVS780. Note that dead cells bind intermediate amounts of Violet Live Cell Caspase Probe. Flow cytometric analysis was performed using a BD LSRFortessa™ Cell Analyzer System. Violet Live Cell Caspase Probe has been tested on mouse (data not shown).

BD Pharmingen™ Violet Live Cell Caspase Probe
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Note: Before staining, confirm that your flow cytometer is capable of exciting the fluorochrome and discriminating the resulting fluorescence.
Preparation
Bring lyophilized probe and 50 μl of fresh cell culture-grade Dimethyl Sulfoxide (DMSO; e.g. Sigma D2650) to room temperature. Add 50 μl of DMSO and vortex solution well. Inspect the solution and repeat vortex until the stock dye has fully dissolved. This is the Stock Solution.
Storage
Upon arrival, store the dry probe desiccated and protected from light at -20°C until use. After reconstitution with DMSO, store the Stock Solution at -20°C in small aliquots. The Stock Solution is stable for at least 3 months post reconstitution with DMSO.
Cytometry Requirements
Violet laser-equipped Flow Cytometers (eg, BD FACSCanto™ II, BD LSRFortessa™, and BD™ LSR II) can be used. This dye can be read out of filters commonly used for BD Horizon™ BV421 (eg, 450/40 nm). Fluorescence compensation is best achieved using a sample of the cells of interest stained with the dye. When designing multicolor panels, we recommend titrating the dye and using the lowest possible concentration that provides adequate resolution of positive and negative populations for the cell type of interest to reduce spillover.
Procedure
Labeling of Cells with BD Pharmingen™ Violet Live Cell Caspase Probe
1. Count cells to determine cell density. Adjust cell density to 1-2 × 10^6 cells/ml in fresh, pre-warmed cell culture media.
a. Adherent cells may be stained either in situ or in suspension after removal from the growth substrate. Cells should be 70% confluent or less when assayed.
b. Note: One "test" is defined as a 500 μL aliquot of cells at 1-2 × 10^6 cells/ml.
2. Add 2 μL of Stock Solution per 1 mL of cell suspension or culture media. For example, for a 500 μL aliquot of cells, add 1 μL of the Stock Solution. Mix thoroughly.
a. In some cases, it may be useful to titrate the probe, as different cell types and different applications can result in variability in staining intensity.
3. Incubate samples for 30-60 minutes at 37°C protected from light.
4. Wash cells once with fresh, pre-warmed media.
5. Resuspend cells in fresh, pre-warmed media and incubate cells for an additional 15-60 minutes at 37°C protected from light to allow unbound probe to diffuse out of the cells.
6. Pellet cells once more by centrifugation and remove the supernatant.
7. Resuspend the cells in 1× Dulbecco's Phosphate Buffered Saline (DPBS) or equivalent.
a. Note: Dead cells bind variable amounts of probe. We recommend co-staining with, eg, BD Via-Probe™ Cell Viability Solution (Cat. No. 555816) in order to distinguish dead cells from live or apoptotic cells.
8. Analyze samples by flow cytometry.
Notes:
1. Each user should determine the optimal concentrations of reagents, cells, and conditions for the assay of interest. We recommend titrating the reagent in early experiments to obtain optimal results.
2. BD Pharmingen™ Violet Live Cell Caspase Probe is compatible with fixation and permeabilization protocols such as those used for BD Phosflow™ staining (eg, Cat. No. 558050, BD Phosflow™ Perm Buffer III) or intracellular cytokine staining (eg, Cat. No. 554714, BD Cytofix/Cytoperm™ Fixation/Permeabilization Kit). However, please note that resolution of positive and negative populations is reduced in fixed and permeabilized cells as compared to live cells. Where possible, it is recommended that samples be analyzed live.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Before staining with this reagent, please confirm that your flow cytometer is capable of exciting the fluorochrome and discriminating the resulting fluorescence.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products


Caspases are critical mediators of apoptosis, and also play roles in other forms of cell death and inflammation. In healthy cells, caspases exist in inactive, pro-enzyme forms. During apoptosis, caspases become activated by cleavage and then proceed to initiate apoptosis. Cells with active caspases can be detected by the BD Pharmingen™ Violet Live Cell Caspase Probe, which contains a fluorochrome-labeled caspase inhibitor. Fluorochrome-labeled caspase inhibitors have three functional domains: (1) a fluorochrome , (2) a 3-amino acid sequence (VAD) that binds to the active site of the activated caspase, and (3) a fluoromethyl ketone (FMK) moiety that allows the probe to irreversibly bind the active caspase. The VAD moiety allows binding of these probes to most of the caspase family (caspase-1, -2, -3, -6, -8, -9, and -10), allowing detection of general caspase activity.
BD Pharmingen™ Violet Live Cell Caspase Probe is excited by the violet laser (with an excitation maximum of 403 nm), and has a fluorescence emission maximum of 454 nm.
Development References (4)
-
Amstad PA, Yu G, Johnson GL, Lee BW, Dhawan S, Phelps DJ. Detection of caspase activation in situ by fluorochrome-labeled caspase inhibitors. Biotechniques. 2001; 31(3):608. (Methodology). View Reference
-
Bedner E, Smolewski P, Amstad P, Darzynkiewicz Z. Activation of caspases measured in situ by binding of fluorochrome-labeled inhibitors of caspases (FLICA): correlation with DNA fragmentation. Exp Cell Res. 2000; 259(1):308-313. (Methodology). View Reference
-
Grabarek J, Amstad P, Darzynkiewicz Z. Use of fluorescently labeled caspase inhibitors as affinity labels to detect activated caspases. Hum Cell. 2002; 15(1):1-12. (Methodology). View Reference
-
Wlodkowic D, Telford W, Skommer J, Darzynkiewicz Z. Apoptosis and beyond: cytometry in studies of programmed cell death. Methods Cell Biol. 2011; 103:55-98. (Methodology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.