-
Your selected country is
Middle East / Africa
- Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?




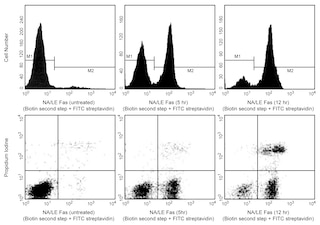
V450 Annexin V: A tool for identifying cells that are undergoing apoptosis. Jurkat T cells (Human T-cell leukemia; ATCC TIB-152) were left untreated (left panel) or treated for 4 hours (right panel) with 6 µM camptothecin. Cells were incubated with V450 Annexin V (Cat. No. 560506) and analyzed by flow cytometry. Untreated cells were primarily V450 Annexin V negative, indicating that they were viable and not undergoing apoptosis (left panel). After a 4 hour treatment with camptothecin, there were two populations of cells: cells undergoing apoptosis (V450 Annexin V positive), and cells that were viable and not undergoing apoptosis (V450 Annexin V negative) (right panel).


BD Horizon™ V450 Annexin V

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
V450 Annexin V is a sensitive probe for identifying apoptotic cells, binding to negatively charged phospholipid surfaces (Kd of ~5 x 10^-2) with a higher affinity for phosphatidylserine (PS) than most other phospholipids. V450 Annexin V binding is calcium-dependent and defined calcium and salt concentrations are required for optimal staining as described in the V450 Annexin V Staining Protocol. Investigators should note that V450 Annexin V flow cytometric analysis on adherent cell types (e.g HeLa, NIH 3T3, etc.) is not routinely tested as specific membrane damage may occur during cell detachment or harvesting. Methods for utilizing Annexin V for flow cytometry on adherent cell types, however, have been previously reported (Casiola-Rosen et al. and van Engelend et al.).
INDUCTION OF APOPTOSIS BY CAMPTOTHECIN
The following protocol is provided as an illustration on how V450 Annexin V may be used on a cell line (Jurkat).
Materials
1. Prepare Camptothecin stock solution (Sigma-Aldrich Cat. No. C-9911): 1 mM in DMSO.
2. Jurkat T cells (ATCC TIB-152).
Procedure
1. Add Camptothecin (final conc. 4-6 µM) to 1 x 10^6 Jurkat cells.
2. Incubate the cells for 4-6 hr at 37°C.
3. Proceed with the V450 Annexin V Staining Protocol to measure apoptosis.
V450 ANNEXIN V STAINING PROTOCOL
V450 Annexin V is used to quantitatively determine the percentage of cells within a population that are actively undergoing apoptosis. It relies on the property of cells to lose membrane asymmetry in the early phases of apoptosis. Annexin V is a calcium-dependent phospholipid-binding protein that has a high affinity for PS, and is useful for identifying apoptotic cells with exposed PS. Propidium Iodide (PI) is a standard flow cytometric viability probe and is used to distinguish viable from nonviable cells.
Reagents
1. V450 Annexin V: Included. Use 5 µl per test.
2. Propidium Iodide (PI): Not Included. PI (Cat.No. 556463) is a convenient, ready-to-use nucleic acid dye. Use up to 10 µl per test of a 50 µg/ml solution.
3. 10× Binding Buffer: Not Included. 0.1 M Hepes (pH 7.4), 1.4 M NaCl, 25 mM CaCl2. Store at 4°C. Alternatively, BD
Pharmingen™ Annexin V Binding Buffer, 10X concentrate (Cat. No. 556454) may be purchased.
Staining
1. Wash cells twice with cold PBS and then resuspend cells in 1× Binding Buffer at a concentration of 1 × 10^6 cells/ml.
2. Transfer 100 µl of the solution (1 × 10^5 cells) to a 5 ml culture tube.
3. Add 5 µl of V450 Annexin V.
4. Add 10 µl PI. The optimal concentration of PI may vary among cell lines where 10 µl of a 50 µg/ml stock is most likely the maximum to be required. Less may yield optimal results in some experimental systems.
5. Gently vortex the cells and incubate for 15 min at RT (25 °C) in the dark.
6. Add 400 µl of 1× Binding Buffer to each tube. Analyze by flow cytometry within 1 hr.
SUGGESTED CONTROLS FOR SETTING UP FLOW CYTOMETRY
The following controls are used to set up compensation and quadrants:
1. Unstained cells.
2. Cells stained with V450 Annexin V (no PI).
3. Cells stained with PI (no V450 Annexin V).
Other Staining Controls:
A cell line that can be easily induced to undergo apoptosis should be used to obtain positive control staining with V450 Annexin V and/or V450 Annexin V and PI. It is important to note that the basal level of apoptosis and necrosis varies considerably within a population. Thus, even in the absence of induced apoptosis, most cell populations will contain a minor percentage of cells that are positive for apoptosis (V450 Annexin V positive, PI negative or V450 Annexin V positive, PI positive).
The untreated population is used to define the basal level of apoptotic and dead cells. The percentage of cells that have been induced to undergo apoptosis is then determined by subtracting the percentage of apoptotic cells in the untreated population from percentage of apoptotic cells in the treated population. Since cell death is the eventual outcome of cells undergoing apoptosis, cells in the late stages of apoptosis will have a damaged membrane and stain positive for PI as well as for V450 Annexin V. Thus the assay does not distinguish between cells that have already undergone an apoptotic cell death and those that have died as a result of necrotic pathway, because in either case the dead cells will stain with both V450 Annexin V and PI.
Product Notices
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
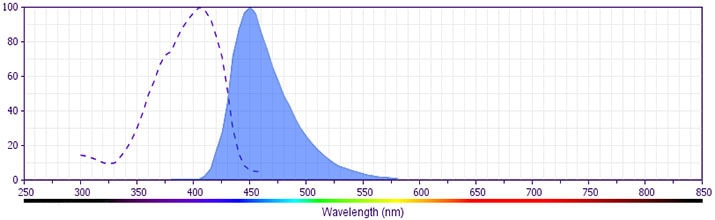
- BD Horizon V450 has a maximum absorption of 406 nm and maximum emission of 450 nm. Before staining with this reagent, please confirm that your flow cytometer is capable of exciting the fluorochrome and discriminating the resulting fluorescence.
- Pacific Blue™ is a trademark of Molecular Probes, Inc., Eugene, OR.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products


Apoptosis is a normal physiological process that occurs during embryonic development and tissue homeostasis maintenance. The apoptotic program is characterized by certain morphological features, including plasma membrane symmetry and detachment, condensation of the cytoplasm and nucleus, and internucleosomal cleavage of DNA. Loss of the plasma membrane is one of the earliest features of apoptosis. In apoptotic cells, phosphatidylserine (PS), a phospholipid membrane component, is translocated from the inner to the outer leaflet of the plasma membrane, thereby exposing PS to the external cellular environment. Annexin V is a 35-36 kDa Ca2+ dependent phospholipid-binding protein that has a high affinity for PS, and binds to cells with exposed PS. Annexin V may be conjugated to fluorochromes including BD Horizon™ V450. This format retains its high affinity for PS and thus serves as a sensitive probe for flow cytometric analysis of cells that are undergoing apoptosis. Since externalization of PS occurs in the earlier stages of apoptosis, V450 Annexin V staining can identify apoptosis at an earlier stage than assays based on nuclear changes such as DNA fragmentation.
V450 Annexin V staining precedes the loss of membrane integrity which accompanies the latest stages of cell death resulting from either apoptotic or necrotic processes. Therefore, staining with V450 Annexin V is typically used in conjunction with a vital dye such as propidium iodide (PI) or 7-Amino-Actinomycin (7-AAD) to allow the investigator to identify early apoptotic cells (PI negative, V450 Annexin V positive). Viable cells with intact membranes exclude PI, whereas the membranes of dead and damaged cells are permeable to PI. For example, cells that are considered viable are both V450 Annexin V and PI negative, cells that are in early apoptosis are V450 Annexin V positive and PI negative, and cells that are in late apoptosis or already dead are both V450 Annexin V and PI positive. This assay does not distinguish between cells that have undergone the apoptotic versus necrotic pathway because dead cells will be stained with both V450 Annexin V and PI. However, when apoptosis is measured over time, cells can often be tracked from V450 Annexin V and PI negative (viable, or no measurable apoptosis), to V450 Annexin V positive and PI negative (early apoptosis, membrane integrity is present) and finally to V450 Annexin V and PI positive (end-stage apoptosis and death). The movement of cells through these three stages suggests apoptosis. In contrast, a single observation indicating that cells are both V450 Annexin V and PI positive, in and of itself, reveals less information about the process by which the cells underwent their demise.
This reagent is conjugated to BD Horizon™ V450, which has been developed for use in multicolor flow cytometry experiments and is available exclusively from BD Biosciences. It is excited by the Violet laser Ex max of 406 nm and has an Em Max at 450 nm. Conjugates with BD Horizon™ V450 can be used in place of Pacific Blue™ conjugates.
Development References (12)
-
Andree HA, Reutelingsperger CP, Hauptmann R, Hemker HC, Hermens WT, Willems GM. Binding of vascular anticoagulant alpha (VAC alpha) to planar phospholipid bilayers. J Biol Chem. 1990; 265(9):4923-4928. (Biology). View Reference
-
Bossy-Wetzel E and Green D. Detection of apoptosis by Annexin V labeling. 2000; 322:15-18. (Biology).
-
Casciola-Rosen L, Rosen A, Petri M, Schlissel M. Surface blebs on apoptotic cells are sites of enhanced procoagulant activity: implications for coagulation events and antigenic spread in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1996; 93(4):1624-1629. (Methodology: Apoptosis, Flow cytometry). View Reference
-
Homburg CH, de Haas M, von dem Borne AE, Verhoeven AJ, Reutelingsperger CP, Roos D. Human neutrophils lose their surface Fc gamma RIII and acquire Annexin V binding sites during apoptosis in vitro. Blood. 1995; 85(2):532-540. (Biology). View Reference
-
Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994; 84(5):1415-1420. (Methodology: Apoptosis, Flow cytometry). View Reference
-
Martin SJ, Reutelingsperger CP, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995; 182(5):1545-1556. (Biology). View Reference
-
O'Brien MC, Bolton WE. Comparison of cell viability probes compatible with fixation and permeabilization for combined surface and intracellular staining in flow cytometry. Cytometry. 1995; 19(3):243-255. (Biology). View Reference
-
Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994; 1197(1):63-93. (Biology). View Reference
-
Schmid I, Krall WJ, Uittenbogaart CH, Braun J, Giorgi JV. Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry. 1992; 13(2):204-208. (Biology). View Reference
-
Van Engeland, Nieland LJW, Ramaekers FCS, Schutte B, and Reutelingsperger CPM. . Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998; 31:1-9. (Biology).
-
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995; 184(1):39-51. (Methodology: Apoptosis, Flow cytometry). View Reference
-
van Engeland M, Ramaekers FC, Schutte B, Reutelingsperger CP. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry. 1996; 24(2):131-139. (Methodology: Apoptosis, Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.