-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- Advanced Training
-
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Human Naive/Memory T Cell Panel
(RUO)



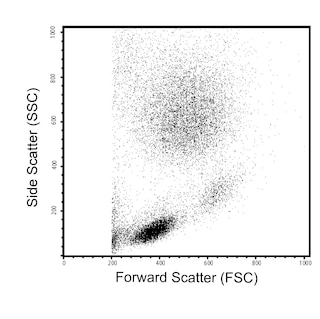
Multicolor staining and flow cytometric analysis of peripheral blood naïve and memory CD4+ T-cells using BD FACSCalibur™ and BD FACSCanto™ Cytometry Systems. Whole blood was stained with a combination of PerCP-Cy™5.5 Mouse anti-Human CD4, FITC Mouse anti-Human CD45RA, and Alexa Fluor® 647 Mouse anti-Human CD197 (CCR7) antibodies for analysis with the BD FACSCalibur™ Cytometer System, or with the addition of APC-H7 Mouse anti-Human CD3 antibody, for analysis with the BD FACSCanto™ Cytometer System. The fluorescent antibodies provided in this kit were each used at 5 μl per test. Red blood cells were then lysed using BD Pharm Lyse™ Lysing Buffer (Cat. No. 555899) and the leukocytes were analyzed. Bivariate dot plots showing the correlated expression patterns of CD45RA versus CD197 (Panels A and B, Lower Plots) were derived from gated events with the light-scattering characteristics of viable CD4+ T lymphocytes (Panel A, Upper Plot, FACSCalibur) or CD4+CD3+ T lymphocytes (Panel B, Upper Plots, FACSCanto II) that were generated by flow cytometric analysis. Quadrants for the dot plots were derived using fluorescence-minus-one (FMO) controls.


BD Pharmingen™ Human Naive/Memory T Cell Panel

Human Naive/Memory T Cell Panel
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
Note: The panel is 50 test size. Extra material for each component for an additional 10 tests is provided for instrument set up purposes.
Multicolor immunofluorescent staining followed by flow cytometric analysis and sorting has led to the phenotypic and functional characterization of multiple peripheral T cell subsets. Three major T cell subsets have been well characterized, i.e., naïve, memory and effector T cells. Naïve T cells have a relatively homogenous cell surface phenotype. They express high levels of CD45RA and the CD197 (CCR7 chemokine receptor). Effector T cells coexpress variable to low levels of CD45RA and no CD197 whereas memory T cells coexpress variable to low levels of CD45RA and high levels of CD197. The Human Naive/Memory T Cell Panel contains fluorescent antibodies (each optimized at 5 μl per test) that are specific for the cell surface antigens: CD45RA, CD197, CD4 and CD3. The channel for detecting Phycoerythrin or Phycoerythrin protein-tandem conjugated proteins has been left open for inclusion of other markers. The panel was designed to standardize the multicolor staining and flow cytometric characterization of the three major CD4+ T cell subsets that arise as a consequence of development or clonal expansion and differentiation driven by antigenic stimulation (eg, in response to allergens, infectious disease or vaccination).
Preparation And Storage
Product Notices
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- The Alexa Fluor®, Pacific Blue™, and Cascade Blue® dye antibody conjugates in this product are sold under license from Molecular Probes, Inc. for research use only, excluding use in combination with microarrays, or as analyte specific reagents. The Alexa Fluor® dyes (except for Alexa Fluor® 430), Pacific Blue™ dye, and Cascade Blue® dye are covered by pending and issued patents.
- Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- Alexa Fluor® 647 fluorochrome emission is collected at the same instrument settings as for allophycocyanin (APC).
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- This APC-conjugated reagent can be used in any flow cytometer equipped with a dye, HeNe, or red diode laser.
- Please observe the following precautions: Absorption of visible light can significantly alter the energy transfer occurring in any tandem fluorochrome conjugate; therefore, we recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to prevent exposure of conjugated reagents, including cells stained with those reagents, to room illumination.
- PerCP-Cy5.5–labelled antibodies can be used with FITC- and R-PE–labelled reagents in single-laser flow cytometers with no significant spectral overlap of PerCP-Cy5.5, FITC, and R-PE fluorescence.
- PerCP-Cy5.5 is optimized for use with a single argon ion laser emitting 488-nm light. Because of the broad absorption spectrum of the tandem fluorochrome, extra care must be taken when using dual-laser cytometers, which may directly excite both PerCP and Cy5.5™. We recommend the use of cross-beam compensation during data acquisition or software compensation during data analysis.
- BD APC-H7 is a tandem conjugate and an analog of APC-Cy7 with the same spectral properties. It has decreased intensity but it is engineered for greater stability and less spillover in the APC channel and consequently offers better performance than APC-Cy7. It has an absorption maximum of approximately 650 nm. When excited by light from a red laser, the APC fluorochrome can transfer energy to the cyanine dye, which then emits at a longer wavelength. The resulting fluorescent emission maximum is approximately 767 nm. BD recommends that a 750-nm longpass filter be used along with a red-sensitive detector such as the Hamamatsu R3896 PMT. As with APC-Cy7 special filters are required when using APC-H7 in conjunction with APC. Note: Although our APC-H7 products demonstrate higher lot-to lot consistency than other APC tandem conjugate products, and every effort is made to minimize the lot-to-lot variation in residual emission from APC, it is strongly recommended that every lot be tested for differences in the amount of compensation required and that individual compensation controls are run for each APC-H7 conjugate.
- Although BD APC-H7 is engineered to minimize spillover to the APC channel and is more stable and less affected by light, temperature, and formaldehyde-based fixatives, compared to other APC-cyanine tandem dyes, it is still good practice to minimize as much as possible, any light, temperature and fixative exposure when working with all fluorescent conjugates.
- Cy is a trademark of GE Healthcare.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products

| Description | Quantity/Size | Part Number | EntrezGene ID |
|---|---|---|---|
| Alexa Fluor® 647 Mouse Anti-Human CD197 (CCR7) | 60 Tests (1 ea) | 51-9007097 | N/A |
| APC-H7 Mouse Anti-Human CD3 | 60 Tests (1 ea) | 51-9007098 | N/A |
| PerCP-Cy™5.5 Mouse Anti-Human CD4 | 60 Tests (1 ea) | 51-9007099 | N/A |
| FITC Mouse Anti-Human CD45RA | 60 Tests (1 ea) | 51-9007100 | N/A |
Development References (5)
-
Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008; 73(11):975-983. (Biology). View Reference
-
Kallies A. Distinct regulation of effector and memory T-cell differentiation. Immunol Cell Biol. 2008; 86(4):325-332. (Biology). View Reference
-
Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004; 22:745-763. (Biology). View Reference
-
Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. 2006; 127(3):274-281. (Biology). View Reference
-
Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008; 8(4):247-258. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.