-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- Advanced Training
-
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
Request a Quote - BD FACSLyric™ Flow Cytometry System
Please fill in the following information and we will get in touch with you regarding your query.
Discover the BD FACSLyric™ Flow Cytometer difference.
- Witness results you have never seen before through high sensitivity and improved resolution
- Streamline your lab workflow through flexibility and automation, enabling efficiency and productivity
- Achieve automated standardization through highly reproducible results and enable collaboration through assay portability
See how the BD FACSLyric™ System can transform your lab.
Get more information from the BD FACSLyric™ Flow Cytometer brochure.

Features
Discover the BD FACSLyric™ Flow Cytometry System—A Next-Generation Flow Cytometer
Built on a foundation of excellence, experience and expertise, the BD FACSLyric™ Flow Cytometry System is a new standard for cell analysis, transforming the way your lab does flow cytometry. As with all BD instruments, the BD FACSLyric™ Flow Cytometry System is backed by 40 years of BD expert training, service and support—so there’s no limit to your potential.
The Power of the BD FACSLyric™ Flow Cytometer
- 4-, 6-, 8-, 10- and 12-color configurations. Onsite upgradeable to adapt to your lab's changing needs
- Up to 3 lasers—blue, red and violet—12 fluorescence channels and 14 parameters
- 35,000 events per second maximum acquisition rate; no limit on number of events acquired
- Automated single-tube QC with BD® CS&T Beads
- Fluorescence compensation required only every 60 days, improving efficiency and productivity
- 21 different loading options: plates or tubes; built-in flexibility with BD FACS™ Universal Loader
- Powered by the BD FACSuite™ Acquisition and Analysis Application, whose password protection, audit trail, electronic signatures and IQ/OQ procedures assist in supporting U.S, FDA 21 CFR Part 11 compliance
- BD FACSLink™ Laboratory Information System (LIS) Interface and BD Assurity Linc™ Remote Systems Management Software streamline your workflow and help improve productivity through seamless integration.
See how you can simplify your workflow using the automated instrument setup and compensation on the BD FACSLyric™ System.

Performance
A new standard delivering outstanding performance and standardization within and between instruments
The BD FACSLyric™ Flow Cytometry System is a high-performance, highly sensitive flow cytometer that demonstrates exceptional resolution and improved separation to make dim and rare cell populations easier to resolve.
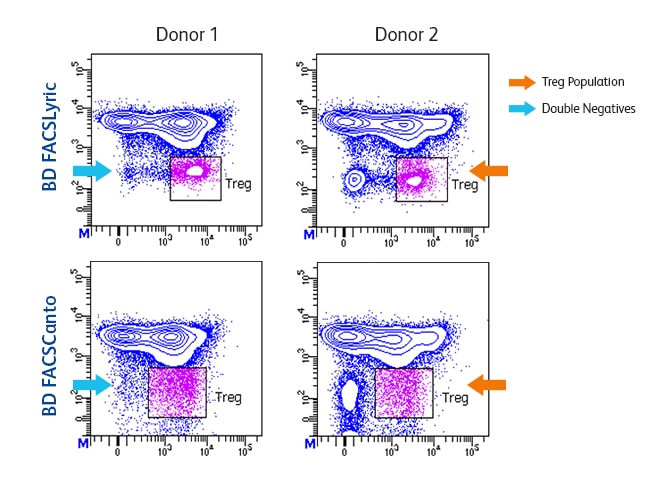
Higher sensitivity makes dim and rare populations easier to resolve.
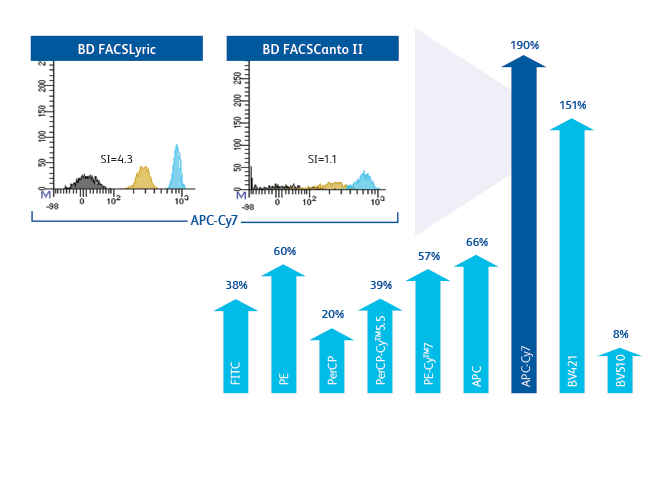
Improvement in stain index of 8–190% across all parameters ensures better separation and enables faster analysis and easier gating.
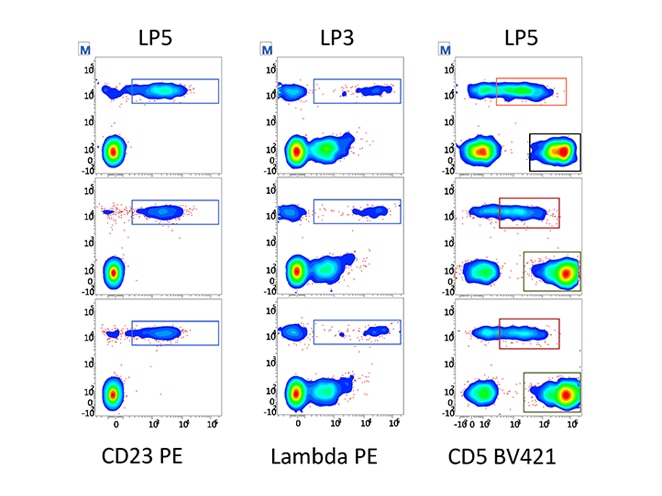
Assay portability simplifies and standardizes instrument setup within your lab and between labs. It also enables the sharing and exchange of data, ideas, and IVD and user-defined protocols within and between institutions.
Highly reproducible results between instruments drive standardization.
| Blue Laser | %CV |
| FITC | 4.2 |
| PE | 4.4 |
| PE-Cy7 | 4.8 |
| PerCP | 10.2 |
| PerCP-Cy5.5 | 7.9 |
| Red Laser | %CV |
| APC | 5.3 |
| APC-Cy7 | 4.1 |
| APC-H7 | 3.9 |
| APC-R700 | 4.2 |
| Violet Laser | %CV |
| BD Horizon™ V450 | 11.1 |
| BD Horizon™ V500 | 11 |
| BD Horizon™ BV605 | 13.6 |
| BD Horizon™ BV711 | 7.5 |
| BD Horizon™ BV786 | 15.3 |
Between-instrument reproducibility of target MFI values on the BD FACSLyric™ System
Lyse/wash assay settings were imported across 15 instruments to show effects of standardization on beads. The CVs of the fluorescence intensity across all channels varies by less than 15.3%. Daily QC with one lot of BD® Cytometer Setup & Tracking Beads was run on fifteen BD FACSLyric™ Flow Cytometers. For each instrument, the PMTV gains were automatically adjusted to meet the target values. BD® FC Beads acquired on each BD FACSLyric™ Instrument. The MFI of positive populations was measured for all parameters across all instruments. The %CV is shown. The data for this internal study were acquired using BD® FC Beads across 15 BD FACSLyric™ Instruments. Greater between-instrument variability could be observed when running biological samples, when using non BD reagents or when comparing fewer instruments.
"BD is positioned with BD® CS&T and BD® FC Bead technology to enable instrument standardization simply from day to day, instrument to instrument, and lab to lab."

"BD is positioned with BD® CS&T and BD® FC Bead technology to enable instrument standardization simply from day to day, instrument to instrument, and lab to lab."
“The consistency and the regulatory compliance of FACSLyric [Flow Cytometer]’s output is what really drew us to the platform. It is currently unmatched by other flow cytometers in its ability to generate reliable, repeatable results across tests and instruments—even for the most complex assays we deal with day to day. We’ve found it particularly beneficial for multiparameter testing for CAR T cells and immunophenotyping.”

“The consistency and the regulatory compliance of FACSLyric [Flow Cytometer]’s output is what really drew us to the platform. It is currently unmatched by other flow cytometers in its ability to generate reliable, repeatable results across tests and instruments—even for the most complex assays we deal with day to day. We’ve found it particularly beneficial for multiparameter testing for CAR T cells and immunophenotyping.”
“The assay portability of BD FACSLyric is the key to efficient method transfer and high data reproducibility. It represents a clear advantage in a regulated environment, where conditions must be met to produce valid results of a guaranteed level of quality. Functional immune cell–based assays and cytokine quantification in preclinical and clinical drug development is a particularly important area where BD FACSLyric offers clear technical advantages.”

“The assay portability of BD FACSLyric is the key to efficient method transfer and high data reproducibility. It represents a clear advantage in a regulated environment, where conditions must be met to produce valid results of a guaranteed level of quality. Functional immune cell–based assays and cytokine quantification in preclinical and clinical drug development is a particularly important area where BD FACSLyric offers clear technical advantages.”
“The BD FACSLyric is a system for everyday work that every technician can use.”

“The BD FACSLyric is a system for everyday work that every technician can use.”


-
Brochure
-
Filter Guide
-
Technical Specifications
-
Product List
-
Product Information Sheets
-
Quick Reference Guide
-
Application Note
-
Posters
-
2016 ESCCA: Simplified Workflow using Automated Setup and Compensation
-
2016 ESCCA: Enhanced Reproducibility of B Cell Assays Using Universal Assay Setup and Dry Reagents
-
2016 ICCS: Analysis of checkpoint Marker Expression Using a 10-color Assay
-
2017 CYTO: Long term CST set-up stability
-
2017 ESCCA: Checkpoint Expression on Immune Cells Using 12-color System
-
2017 ESCCA: 12-color Activation and Homing T reg Panel
-
2017 ICCS: Automated Instrument Setup and Compensation
-
2018 ESCCA: IP CD34 UK NEQAS Samples
-
2018 ESCCA: IP CLL UK NEQAS Samples
-
2019 ICCS: Development of a Dried-down, Multicolor Reagent Solution for Enhanced Flow-cytometric Applications
-
Data Sheets
BD FACSLyric™ Flow Cytometers are Class 1 Laser Products.
The BD FACSLyric™ Flow Cytometer is For Research Use Only. Not for use in diagnostic or therapeutic procedures. Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.
