Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


.png)

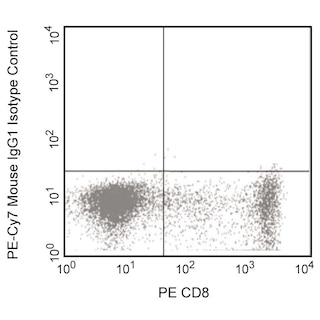
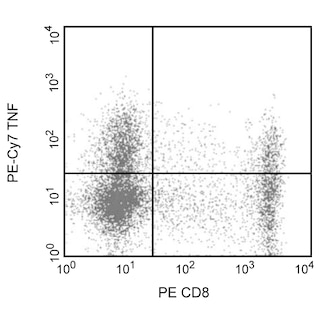
Expression of TNF by stimulated human peripheral blood mononuclear cells (PBMC). Human PBMC were stimulated for 4 hrs with PMA (5 ng/ml, Sigma) and Ionomycin (500 ng, Sigma) in the presence of Brefeldin A (Cat. No. 555029). Cells were harvested, fixed, permeabilized and stained with PE mouse anti-human CD8 (Cat. No. 555367) and either PE-Cy7 mouse anti-human TNF antibody (Cat. No.557647/560923/560678, left panel) or PE-Cy™7 Mouse IgG1 κ Isotype Control (Cat. No. 557646, right panel). To demonstrate specificity of staining, the binding of PE-Cy™7 Mouse Anti-Human TNF was blocked by the preincubation of the conjugated antibody with molar excess of recombinant human TNF (0.25 µg,Cat. No. 554618) and the fixed/permeabilized cells with an excess of Purified Mouse Anti-Human TNF (5 µg, Cat. No. 554510) (data not shown) prior to staining. Quadrant markers were set based on the autofluorescence and isotype controls.
.png)

BD Pharmingen™ PE-Cy™7 Mouse Anti-Human TNF
.png)
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
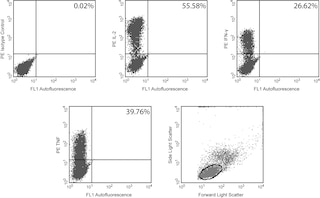
Immunofluorescent Staining and Flow Cytometric Analysis: The MAb11 antibody is useful for intracellular immunofluorescent staining and flow cytometric analysis to identify and enumerate TNF producing cells within mixed cell populations. The FITC-, PE-, APC-, and PE-Cy7-conjugated MAb11 antibodies (Cat. No. 554512, 554513 , 554514, and557647, respectively) are especially suitable for these studies (see image). For optimal immunofluorescent staining with flow cytometric analysis, this anti-cytokine antibody should be used at 5 µl/test The staining technique and use of blocking controls are described in detail by C. Prussin and D. Metcalfe.3 For specific methodology, please visit our website, http://www.bdbiosciences.com/us/s/resources, and go to the protocols section under "Cytokines (Intracellular Staining)" or "Intracellular Flow".
ELISA Detection: The biotinylated MAb11 antibody (Cat. No. 554511) can be used as the detection antibody in a sandwich ELISA for measuring human TNF protein levels in conjunction with clone MAb1 (Cat. No. 551220; purified mouse anti-human TNF) as the capture antibody and recombinant human TNF (Cat. No. 554618) as the standard. For specific methodology, please visit the protocols section under "ELISA and ELISPOT" at our website, http://www.bdbiosciences.com/us/s/resources.
Product Notices
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- An isotype control should be used at the same concentration as the antibody of interest.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
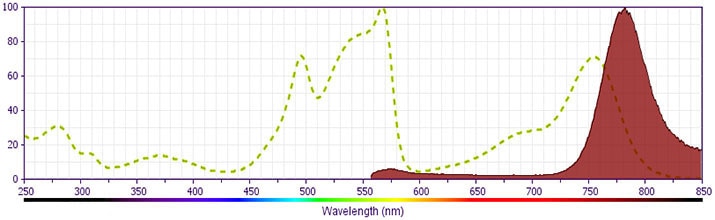
- PE-Cy7 is a tandem fluorochrome composed of R-phycoerythrin (PE), which is excited by 488-nm light and serves as an energy donor, coupled to the cyanine dye Cy7, which acts as an energy acceptor and fluoresces maximally at 780 nm. PE-Cy7 tandem fluorochrome emission is collected in a detector for fluorescence wavelengths of 750 nm and higher. Although every effort is made to minimize the lot-to-lot variation in the efficiency of the fluorochrome energy transfer, differences in the residual emission from PE may be observed. Therefore, we recommend that individual compensation controls be performed for every PE-Cy7 conjugate. PE-Cy7 is optimized for use with a single argon ion laser emitting 488-nm light, and there is no significant overlap between PE-Cy7 and FITC emission spectra. When using dual-laser cytometers, which may directly excite both PE and Cy7, we recommend the use of cross-beam compensation during data acquisition or software compensation during data analysis.
- Please observe the following precautions: Absorption of visible light can significantly alter the energy transfer occurring in any tandem fluorochrome conjugate; therefore, we recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to prevent exposure of conjugated reagents, including cells stained with those reagents, to room illumination.
- Warning: Some APC-Cy7 and PE-Cy7 conjugates show changes in their emission spectrum with prolonged exposure to formaldehyde. If you are unable to analyze fixed samples within four hours, we recommend that you use BD™ Stabilizing Fixative (Cat. No. 338036).
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Cy is a trademark of GE Healthcare.
- Species cross-reactivity detected in product development may not have been confirmed on every format and/or application.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products



The MAb11 monoclonal antibody specifically binds to human tumor necrosis factor (TNF, also known as TNF-α) protein. TNF is an efficient juxtacrine, paracrine and endocrine mediator of inflammatory and immune functions. It regulates the growth and differentiation of a variety of cell types. TNF is cytotoxic for transformed cells when in conjunction with IFN-γ. It is secreted by activated monocytes/macrophages and other cells such as B cells, T cells and fibroblasts. The immunogen used to generate the MAb11 hybridoma was recombinant human TNF. The MAb11 antibody has been reported to crossreact with Rhesus Macaque TNF.
Development References (5)
-
Jason J, Larned J. Single-cell cytokine profiles in normal humans: comparison of flow cytometric reagents and stimulation protocols. J Immunol Methods. 1997; 207(1):13-22. (Biology). View Reference
-
Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995; 188(1):117-128. (Biology). View Reference
-
Sander B, Andersson J, Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991; 119:65-93. (Biology). View Reference
-
Sopper S, Stahl-Hennig C, Demuth M, Johnston IC, Dorries R, ter Meulen V. Lymphocyte subsets and expression of differentiation markers in blood and lymphoid organs of rhesus monkeys. Cytometry. 1997; 29(4):351-362. (Biology). View Reference
-
Verdier F, Aujoulat M, Condevaux F, Descotes J. Determination of lymphocyte subsets and cytokine levels in cynomolgus monkeys. Toxicology. 1995; 105(1):81-90. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.