Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD™ FITC Avidin
(RUO (GMP))

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Store vials at 2°C–8°C. Conjugated forms should not be frozen. Protect from exposure to light. Each reagent is stable until the expiration date shown on the bottle label when stored as directed.
Avidin is prepared chromatographically from egg white.
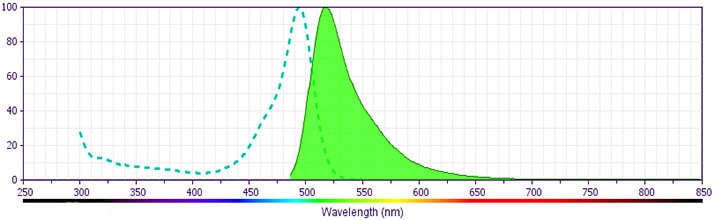
Development References (5)
-
Centers for Disease Control. Update: universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in healthcare settings. MMWR. 1988; 37:377-388. (Biology).
-
Clinical and Laboratory Standards Institute. 2005. (Biology).
-
Green NM. Avidin. Adv Protein Chem. 1975; 29:85-133. (Biology).
-
Parks DR, Lanier LL, Herzenberg LA. Weir DM, ed. Handbook of Experimental Immunology. Oxford: Blackwell Scientific Publications; 1986:29.
-
Wood GS, Warnke RA. Suppression of endogenous avidin-binding activity and its relevance to biotin-avidin detection systems. J Histochem Cytochem. 1981; 29:1196. (Biology).
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Although not required, these products are manufactured in accordance with Good Manufacturing Practices.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.