Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Leucocount™ Kit with BD Trucount™ Tubes
(IVD)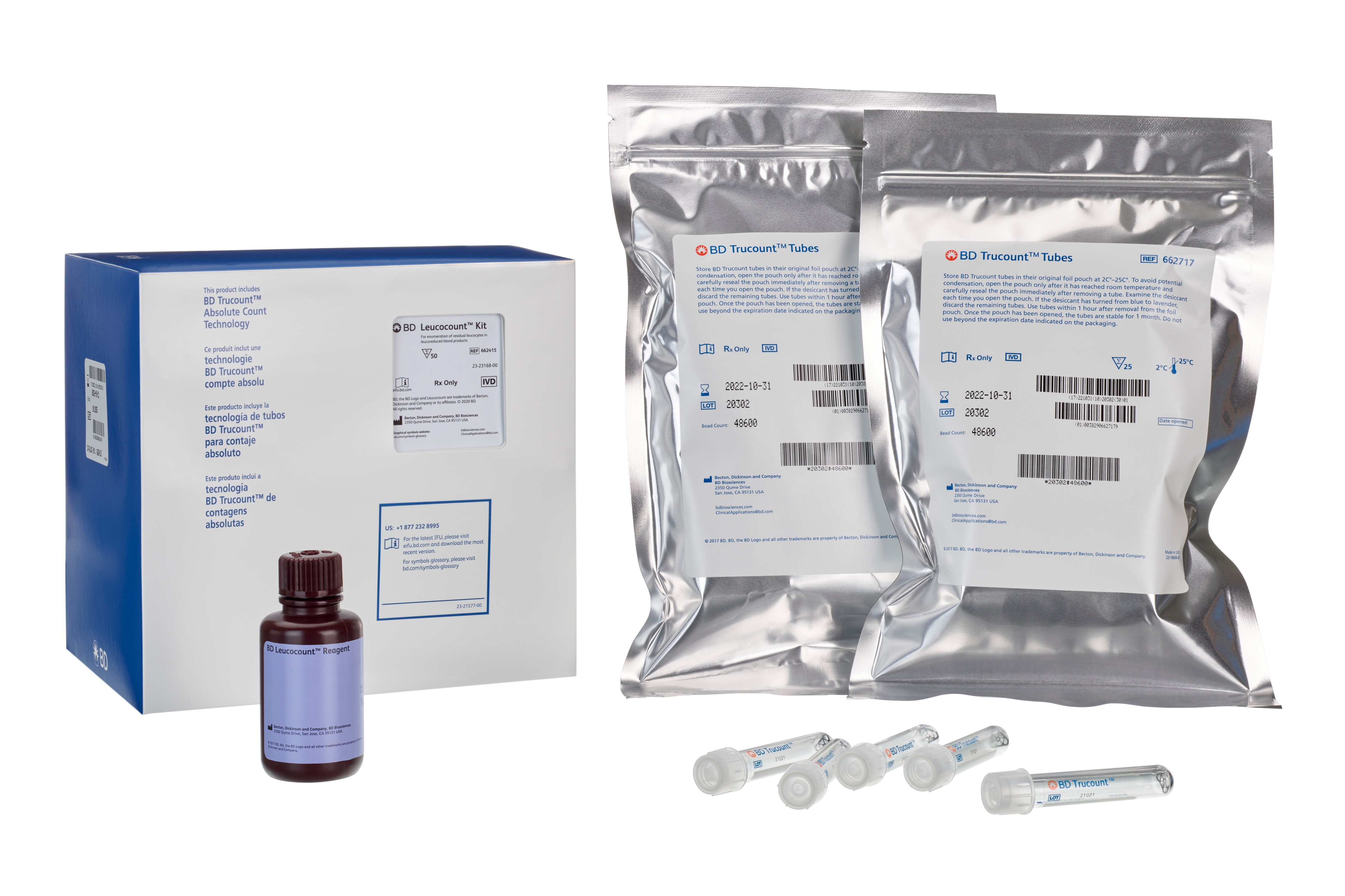

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
The BD Leucocount™ kit consists of BD Leucocount™ reagent (propidium iodide fluorescent dye) and BD Trucount™ tubes and is intended for use with the BD FACSLyric™, BD FACSCalibur™, BD FACSort™, BD FACScan™, and BD FACSVia™ flow cytometer systems, or a flow cytometer equipped with a 488-nm laser able to threshold on propidium iodide fluorescence, for enumerating residual white blood cells (rWBCs) in leucoreduced blood products.
Preparation And Storage
• Store BD Leucocount reagent at 2°C– 8°C. Do not use after the expiration date shown on the label. • Avoid unnecessary exposure of the reagent to light. • Do not freeze the reagents or expose them to direct light during storage or incubation with cells. Keep the reagent vials dry. • Store BD Trucount tubes in their original foil pouch at 2°C–25°C. To avoid potential condensation, open the pouch only after it has reached room temperature and carefully reseal the pouch immediately after removing a tube. Examine the desiccant each time you open the pouch. If the desiccant has turned from blue to lavender, discard the remaining tubes. Use tubes within 1 hour after removal from the foil pouch. Once the pouch has been opened, the tubes are stable for 1 month. Do not use beyond the expiration date indicated on the packaging.
| Description | Quantity/Size | Part Number | EntrezGene ID |
|---|---|---|---|
| Leucocount Reagent | 50 Tests (1 ea) | 91-1211 | N/A |
| BD Trucount tubes (25 tubes) | 1 Each (2 ea) | 91-0786 | N/A |
Development References (11)
-
Bull BS, Breton-Corius J. Beutler E, Lichtman MA, Coller BS, Kripps TJ, ed. Williams Hematology. New York, NY: McGraw-Hill, Inc ; 1995:349.
-
Center for Biologics Evaluation and Research. Recommendations and Licensure Requirements for Leukocyte-Reduced Blood Products. 1996. (Biology).
-
Centers for Disease Control. Update: universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in healthcare settings. MMWR. 1988; 37:377-388. (Biology).
-
Clinical and Laboratory Standards Institute. 2005. (Biology).
-
Dumont LJ, Dzik WH, Rebulla P, Brandwein H Practical guidelines for process validation and process control of white cell-reduced blood components: report of the Biomedical Excellence for Safer Transfusion (BEST) Working Party of the International Society of Blood Transfusion (ISBT). Transfusion. 1996; 11-20. (Biology).
-
Rebulla P, Porretti L, Bertolini F, et al. White cellreduced red cells prepared by filtration: a critical evaluation of current filters and methods for counting residual white blood cells. Transfusion. 1993; 33:128-133. (Biology).
-
Sniecinski I, O'Donnell MR, Nowicki B, Hill LR. Prevention of refractoriness and HLAalloimmunization using filtered blood products. Blood. 1988; 71:1402-1407. (Biology).
-
VanCott, J, H Staats, et al. Regulation of mucosal and systematic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J Immunol. 1996; 156:1504-1514. (Biology).
-
Venglen-Tyler V, ed. Leukoreduction of RBC and platelet units. American Association of Blood Banks. 1996; 722-725. (Biology).
-
Wenz B, Gurtlinger K, O'Toole A, Dugan E. Preparation of granulocyte-poor red blood cells by microaggregate filtration. A simplified method to minimize febrile transfusion reactions. Vox Sang. 1980; 39:282-287. (Biology).
-
de Graan-Hentzen YCE, Gratama JW, Mudde GC, et al. Prevention of primary cytomegalovirus infection in patients with hematologic malignancies by intensive white cell depletion of blood products. Transfusion. 1989; 29:757-760. (Biology).
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For In Vitro Diagnostics Use.
Refer to manufacturer's instructions for use and related User Manuals and Technical Data Sheets before using this product as described.
Comparisons, where applicable, are made against older BD technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.