Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
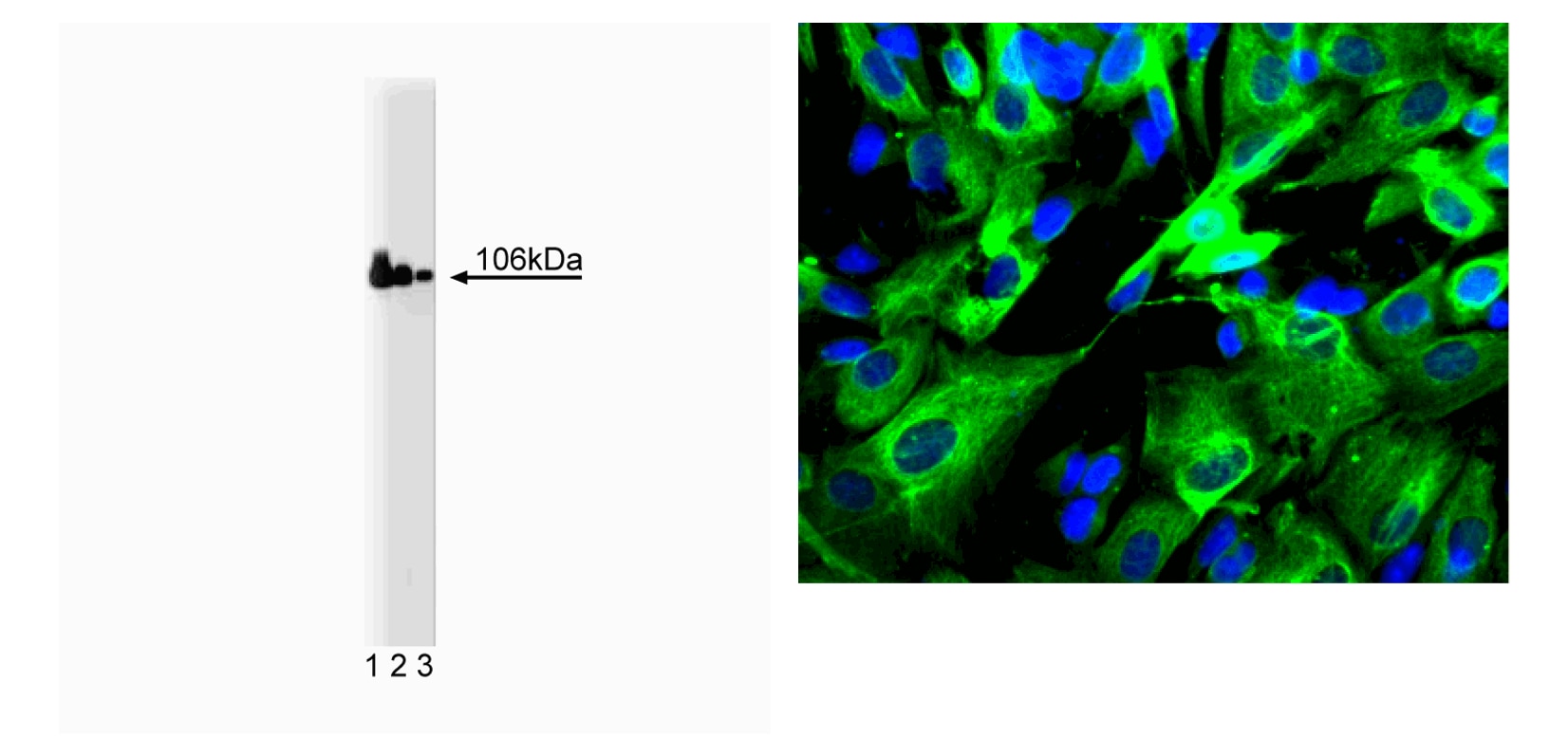



Western blot analysis of Adaptin β on a Jurkat cell lysate (Left Panel). Jurkat cell lysate (Human T-cell leukemia; ATCC TIB-152) was stained with Purified Mouse Anti-Adaptin β (Cat. No. 610381/610382) at the following dilutions: 1:5000 (lane 1), 1:10,000 (lane 2), 1:20,000 (lane 3). Adaptin β expression was visualized with HRP Goat Anti-Mouse Ig (Cat. No. 554002), and expressed as a 106 kDa band. Immunofluorescent staining of SK-N-SH cells (Right Panel). Cells (Human neuroblastoma; ATCC HTB-11) were seeded in a collagen coated 384-well imaging plate at ~ 8,000 cells per well. After overnight incubation, cells were stained using the Triton-X 100 fix/perm protocol (see Recommended Assay Procedure) and Purified Mouse Anti-Adaptin β. The second step reagent was Alexa Fluor® 488 goat anti-mouse Ig (Invitrogen). The image was taken on a BD Pathway™ 855 or 435 Bioimager using a 20x objective. This antibody also stained SH-SY5Y (Human neuroblastoma; ATCC CRL-2266), C6 (Rat glioma; ATCC CCL-107), U-87 MG (Human glioblastoma cells; ATCC HTB-14) and U-373 cells (Human glioblastoma cells; ATCC HTB-17; discontinued) using both the Triton-X 100 and methanol fix/perm protocols (see Recommended Assay Procedure).


BD Transduction Laboratories™ Purified Mouse Anti-Adaptin β

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Bioimaging
1. Seed the cells in appropriate culture medium at ~10,000 cells per well in a 96-well imaging plate and culture overnight.
2. Remove the culture medium from the wells, and fix the cells by adding 100 μl of BD Cytofix™ Fixation Buffer (Cat. No. 554655) to each well. Incubate for 10 minutes at room temperature (RT).
3. Remove the fixative from the wells, and permeabilize the cells using either BD Perm Buffer III, 90% methanol, or Triton™ X-100:
a. Add 100 μl of -20°C 90% methanol or Perm Buffer III (Cat. No. 558050) to each well and incubate for 5 minutes at RT.
OR
b. Add 100 μl of 0.1% Triton™ X-100 to each well and incubate for 5 minutes at RT.
4. Remove the permeabilization buffer, and wash the wells twice with 100 μl of 1× PBS.
5. Remove the PBS, and block the cells by adding 100 μl of BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) to each well. Incubate for 30 minutes at RT.
6. Remove the blocking buffer and add 50 μl of the optimally titrated primary antibody (diluted in Stain Buffer) to each well, and incubate for 1 hour at RT.
7. Remove the primary antibody, and wash the wells three times with 100 μl of 1× PBS.
8. Remove the PBS, and add the second step reagent at its optimally titrated concentration in 50 μl to each well, and incubate in the dark for 1 hour at RT.
9. Remove the second step reagent, and wash the wells three times with 100 μl of 1× PBS.
10. Remove the PBS, and counter-stain the nuclei by adding 200 μl per well of 2 μg/ml Hoechst 33342 (e.g., Sigma-Aldrich Cat. No. B2261) in 1× PBS to each well at least 15 minutes before imaging.
11. View and analyze the cells on an appropriate imaging instrument.
Bioimaging & Western Blot: For more detailed information please refer to "Cellular Imaging" or "Cell Biology (WB, IP, IHC, IF)" at our website: http://www.bdbiosciences.com/us/s/resources
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- This antibody has been developed and certified for the bioimaging application. However, a routine bioimaging test is not performed on every lot. Researchers are encouraged to titrate the reagent for optimal performance.
- Sodium azide is a reversible inhibitor of oxidative metabolism; therefore, antibody preparations containing this preservative agent must not be used in cell cultures nor injected into animals. Sodium azide may be removed by washing stained cells or plate-bound antibody or dialyzing soluble antibody in sodium azide-free buffer. Since endotoxin may also affect the results of functional studies, we recommend the NA/LE (No Azide/Low Endotoxin) antibody format, if available, for in vitro and in vivo use.
- Species cross-reactivity detected in product development may not have been confirmed on every format and/or application.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Triton is a trademark of the Dow Chemical Company.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products


Sorting of integral membrane proteins at various stages of the endocytic and secretory pathways is mediated by vesicular trafficking between a variety of organelles. Two sorting signals are tyrosine-based and dileucine-based signals that interact with heterotetrameric adaptor protein complexes (AP-1, AP-2, AP-3, and AP-4), which are associated with the vesicle coats. These coatomers contain two large Adaptin proteins (γ, α, δ, or ε and β1, β2, β3, or β4, respectively) that are noncovalently linked to one medium chain (µ1, µ2, µ3, or µ4) and one small chain (σ1, σ2, σ3, or σ4). The AP-1 and AP-3 complexes are involved in protein sorting from the TGN and endosomes, while AP-2 adaptor complexes are involved in clathrin-mediated endocytosis. β Adaptin subunits (β1, β2, β3, β4) lack sequence homology to adaptins α, γ, δ, and ε, but all of these subunits share a similar domain structure. Adaptin β1 (also known as Adaptin β') and β2 (also known as Adaptin β) have 83% amino acid identity and are found in the AP1 and AP2 complexes, respectively.
Development References (5)
-
Kirchhausen T, Nathanson KL, Matsui W. Structural and functional division into two domains of the large (100- to 115-kDa) chains of the clathrin-associated protein complex AP-2. Proc Natl Acad Sci U S A. 1989; 86(8):2612-2616. (Biology). View Reference
-
Laporte SA, Oakley RH, Zhang J. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci U S A. 1999; 96(7):3712-3717. (Biology: Immunofluorescence). View Reference
-
Naga Prasad SV, Laporte SA, Chamberlain D, Caron MG, Barak L, Rockman HA. Phosphoinositide 3-kinase regulates beta2-adrenergic receptor endocytosis by AP-2 recruitment to the receptor/beta-arrestin complex. J Cell Biol. 2002; 158(3):563-575. (Biology: Western blot). View Reference
-
Ponnambalam S, Robinson MS, Jackson AP, Peiperl L, Parham P. Conservation and diversity in families of coated vesicle adaptins. J Biol Chem. 1990; 265(9):4814-4820. (Biology). View Reference
-
Ros-Baro A, Lopez-Iglesias C, Peiro S. Lipid rafts are required for GLUT4 internalization in adipose cells. Proc Natl Acad Sci U S A. 2001; 98(21):12050-12055. (Biology: Western blot). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.