Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


Anti-Myeloperoxidase (MPO) PE
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
The antibody reagent is stable until the expiration date shown on the label when stored at 2° to 8°C. Do not use after the expiration date. Do not freeze the reagent or expose it to direct light during storage or incubation with cells. Keep the outside of the reagent vial dry.
Do not use the reagent if you observe any change in appearance. Precipitation or discoloration indicates instability or deterioration.
Anti-human myeloperoxidase (MPO) is intended for in vitro diagnostic use in the identification of cells expressing MPO antigen, using a BD FACS™ brand flow cytometer. The flow cytometer must be equipped to detect light scatter and the appropriate fluorescence, and be equipped with appropriate analysis software (such as BD CellQuest™ or BD LYSYS™ II software) for data acquisition and analysis. Refer to your instrument user’s guide for instructions.
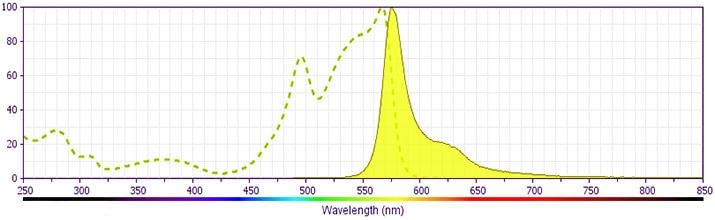
Development References (16)
-
Audrain MA, Baranger TA, Moguilevski N, et al. Anti-native and recombinant myeloperoxidase monoclonals and human autoantibodies. Clin Exp Immunol. 1997; 107(1):127-134. (Biology). View Reference
-
Centers for Disease Control. Update: universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in healthcare settings. MMWR. 1988; 37:377-388. (Biology).
-
Clinical Applications of Flow Cytometry: Quality Assurance and Immunophenotyping of Lymphocytes: Approved Guideline. H42-A2. 2007. (Biology).
-
Consensus protocol for the flow cytometric immunophenotyping of hematopoietic malignancies. Rothe G, Schmitz G. Leukemia. 1996; 10:877-895. (Biology).
-
Groeneveld K, te Marvelde JG, van den Beemk MW, Hooijkaas H, van Dongen JJ. Flow cytometric detection of intracellular antigens for immunophenotyping of normal and malignant leukocytes. Leukemia. 1996; 10:1383-1389. (Biology).
-
Grégoire C, Welch H, Astarie-Dequeker C, Maridonneau-Parini I. Expression of azurophil and specific granule proteins during differentiation of NB4 cells in neutrophils. J Cell Physiol. 1998; 175:203-210. (Biology).
-
Jackson AL, Warner NL. Rose NR, Friedman H, Fahey JL, ed. Manual of Clincial Laboratory Immunology, Third Edition. Washington DC: American Society for Microbiology; 1986:226-235.
-
Knapp W, Strobl H, Majdic O. Flow cytometric analysis of cell-surface and intracellular antigens in leukemia diagnosis. Cytometry. 1994; 18(4):187-198. (Biology). View Reference
-
Knowles DM. Knowles DM, Thompson DD, ed. Neoplastic Hematopathology. Philadelphia, PA: Williams & Wilkins; 2001.
-
Lanza F, Latorraca A, Moretti S, Castagnari B, Ferrari L, Castoldi G. Comparative analysis of different permeabilization methods for the flow cytometry measurement of cytoplasmic myeloperoxidase and lysozyme in normal and leukemic cells. Cytometry. 1997; 30(3):134-144. (Biology). View Reference
-
NCCLS document. 2001. (Biology).
-
Nauseef WM, Malech HL. Analysis of the peptide subunits of human neutrophil myeloperoxidase. Blood. 1986; 5:1504-1507. (Biology).
-
Nguyen PL, Olszak I, Harris NL, Preffer FI. Myeloperoxidase detection by three-color flow cytometry and by enzyme cytochemistry in the classification of acute leukemia. Am J Clin Pathol. 1998; 110:122-124. (Biology).
-
Peffault de Latour R, Legrand O, Moreau D, et al. Comparison of flow cytometry and enzyme chtochemistry for the detection of myeloperoxydase in acute myeloid leukaemia: interests of a new positivity threshold. Br J Haematol. 2003; 122:211-216. (Biology).
-
Stelzer GT, Marti G, Hurley A, McCoy PJ, Lovett EJ, Schwartz A. US-Canadian consensus recommendations on the immunophenotypic analysis of hematologic neoplasia by flow cytometry: standardization and validation of laboratory procedures. Cytometry. 1997; 30:214-230. (Biology).
-
Tobler A, Miller CW, Johnson KR, Selsted ME, Rovera G, Koeffler HP. Regulation of gene expression of myeloperoxidase during myeloid differentiation. J Cellular Physiol. 1988; 136:215-225. (Biology).
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For In Vitro Diagnostic Use.
23-22942-00
![]()
Documents are subject to revision without notice. Please verify you have the correct revision of the document, and always refer back to BD's eIFU website for the latest and most up to date information.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.