Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Stemflow™ Human Neural Lineage Analysis Kit
(RUO)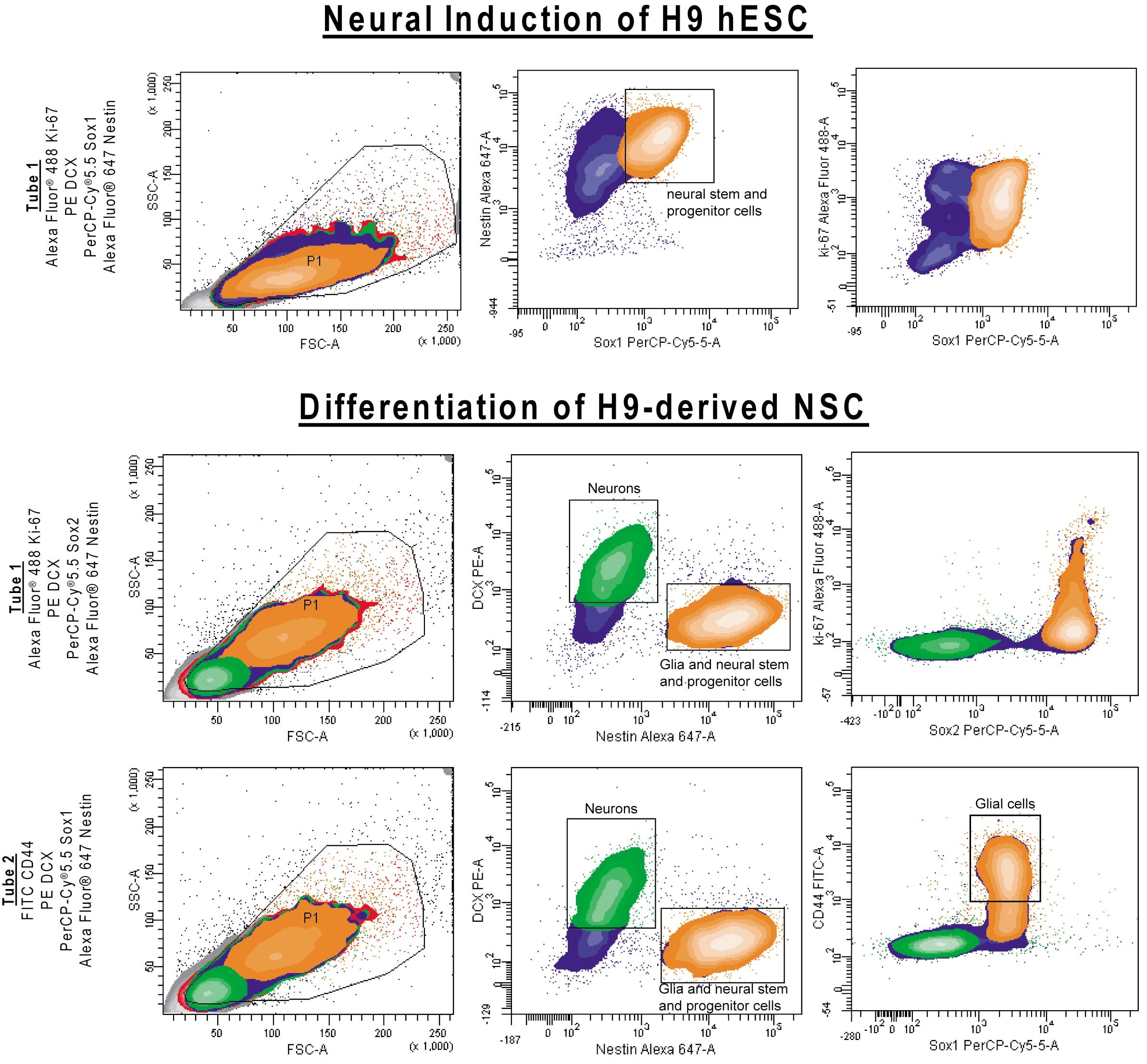





Neural Induction of H9 hESC: H9 Human embryonic stem cells (hESC) (Wicell Madison, WI) were cultured on mouse embryonic fibroblasts in hESC medium (DMEM:F12, 20% Knockout Serum Replacement (KOSR), non-essential amino acids, 20 mM glutamine) (Invitrogen), 1X penicillin/streptomycin (Lonza), bFGF (Cat. No. 354060). Cell cultures were treated with Dispase (Cat. No. 354235) and cell colonies were scraped and placed in hESC medium without FGF in low adhesion plates to form embroid bodies (EB). After 4 days the EBs were plated on BD™ Matrigel-coated plates (Cat. No. 354277) and placed in neural induction medium (DMEM:F12 (Invitrogen), 1X ITS supplement (Sigma), 250 ng/ml recombinant Noggin (R&D Systems) and penicillin/streptomycin (Lonza) and cultured for an additional 15 days. Differentiation of H9-derived NSC: H9 hESC-derived NSC were differentiated in neural differentiation medium (DMEM:F12, 0.5X B27, 0.5X N2 (Invitrogen), 1X penicillin/streptomycin (Lonza), 20 ng/ml BDNF, 20ng/ml GDNF (both from Peprotech) and 0.5 mM dibutyryl cyclic AMP (Sigma) for 28 days.

Differentiation of H9-derived NSC: H9 hESC-derived NSC were differentiated in neural differentiation medium (DMEM:F12, 0.5X B27, 0.5X N2 (Invitrogen), 1X penicillin/streptomycin (Lonza), 20 ng/ml BDNF, 20ng/ml GDNF (both from Peprotech) and 0.5 mM dibutyryl cyclic AMP (Sigma) for 11 days. Culture medium was replaced with AGM™ Astrocyte Growth Medium (Lonza) for an additional 16 days.


BD Stemflow™ Human Neural Lineage Analysis Kit

BD Stemflow™ Human Neural Lineage Analysis Kit

Human Neural Lineage Analysis Kit
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
The BD Stemflow™ Human Neural Lineage Analysis Kit contains a combination of mouse monoclonal antibody conjugates for the analysis of neural differentiation cultures. The antibody specificities that are provided in this kit and the neural cell populations they can identify are listed in the table below. The antibody components of this kit can be used individually or in combinations of up to four. All Antibodies in this kit have been verified by western blot and image analysis. Additional antibody formats that can enable more complex panels are available at www.bdbiosciences.com. The kit also includes BD Cytofix™ Fixation Buffer and BD Phosflow™ Perm Buffer III.
Kit Components
Component Description Size Vol. Per Test Storage Buffer
51-9007227 PerCP-Cy™5.5 Mouse Anti-Sox2 25 Test 5 µl Aqueous buffered solution containing
BSA and ≤ 0.09% sodium azide
51-9007228 Alexa Fluor® 647 Mouse Anti-GFAP 25 Test 5 µl Aqueous buffered solution containing
BSA and ≤ 0.09% sodium azide
51-9007229 PE Mouse Anti-Doublecortin 25 Test 5 µl Aqueous buffered solution containing
BSA and ≤ 0.09% sodium azide
51-9007230 Alexa Fluor® 647 Mouse Anti-Nestin 25 Test 5 µl Aqueous buffered solution containing
BSA and ≤ 0.09% sodium azide
51-9007231 Alexa Fluor® 488 Mouse Anti-Human Ki-67 25 Test 5 µl Aqueous buffered solution containing
BSA and ≤ 0.09% sodium azide
51-9007232 PerCP-Cy™5.5 Mouse Anti-Human Sox1 25 Test 5 µl Aqueous buffered solution containing
BSA and ≤ 0.09% sodium azide
51-9007233 FITC Mouse Anti-Human CD44 25 Test 5 µl Aqueous buffered solution containing
BSA and ≤ 0.09% sodium azide
51-9006607 Perm Buffer III 50 ml
51-9006276 Fixation Buffer 50 ml
Specificity Clone Molecule Neural cell population identified
CD44 G44-26 H-CAM Glial cells, astrocytes, astrocyte precursors
Ki-67 B56 Nuclear proliferation marker All proliferating cell types
Doublecortin 30/Doublecortin Neuroflilament Immature post-mitotic neurons
Sox2 O30-678 Transcription factor Glial cells, embryonic and neural stem cells
Sox1 N23-844 Transcription factor Glial cells and neural stem cells
GFAP 1B4 Filament Astrocytes
Nestin 25/Nestin Filament Glial cells, astrocytes, embryonic and neural stem cells
Preparation And Storage
Recommended Assay Procedures
(1) Detach cells of interest from the culture dish. Investigators are encouraged to detach cells at 37°C using Accutase™ Cell Detachment Solution (Cat. No. 561527). Detachment can take up to 45 minutes with mild to moderate triturating of the cell suspension.
(2) Wash the cells in 1X PBS and filter using a 70 μm Falcon® cell strainer (Corning Cat. No. 352350).
(3) Resuspend the cells in BD Cytofix™ Fixation Buffer (Cat. No. 554655) and incubate at room temperature for 20 minutes in the dark. Recommended volumes for fixing cells is 1 ml of BD Cytofix™ Fixation Buffer per 10 million cells with a minimum volume of 200 µl.
(4) Wash the cells twice with 1X PBS. If investigators wish to continue at a later time, cells may be frozen and stored in a solution of 10% DMSO + 90% FBS at -80°C using standard methods for the cryopreservation of live cells.
(5) Vortex to loosen the cells and then permeabilize the cells by slowly adding ice cold BD Phosflow™ Perm Buffer III (Cat. No. 558050) while vortexing. Recommended volumes for permeabilizing cells is 1 ml of BD Phosflow™ Perm Buffer III per 10 million cells with a minimum volume of 1 mL.
(6) Incubate on ice for 30 minutes, or alternatively, the cells can be stored in BD Phosflow™ Perm Buffer III for up to 6 months at -20°C.
(7) Wash the cells twice with BD Pharmingen™ Stain Buffer (FBS) (Cat. No.554656) and resuspend at 5 - 10 million cells/ml in BD Pharmingen™ Stain Buffer (FBS) . Alternatively, 1X Washing/Staining Solution (1 x PBS,1% FCS, and 0.09% sodium azide) may be used.
(8) Add 100 µl cells into appropriately labeled 12 x 75mm polystyrene tubes.
(9) Add panel of antibody conjugates that you wish to use ( 5µl/test of each antibody conjugate) and incubate in the dark for 30 minutes.
(10) Wash twice with 2 ml BD Pharmingen™ Stain Buffer (FBS).
(11) Resuspend sample in appropriate volume (350-400µl) of BD Pharmingen™ Stain Buffer (FBS) to run on a flow cytometer.
Kit Considerations:
Choosing a Cell Detachment Enzyme: Investigators are encouraged to use Accutase™ Cell Detachment Solution (Cat. No. 561527), as cell death with this detachment method has been observed to be minimal. Other methods can be used depending on the confluency and number of cells, such as with 0.05% trypsin.
Fixation and Permeabilization Methods: The components of this kit are all compatible with each other. However if additional specificities are included, those antibody conjugates must be verified to work with the fix/perm buffers included in this kit.
Instrument Set Up: BD™ CompBead Plus particles (Cat. No. 560497 or 560499) may be beneficial in setting up the experiment on a flow cytometer. Investigators are encouraged to set up PMT voltages with the BD™ CompBead Plus particles and then checking to ensure the voltage settings are appropriate by running a stained sample of cells before running the entire experiment.
Data Analysis: A cluster based gating approach is recommended when analyzing data. Backgating to the scatter profile may be useful as the neurons have a distinct scatter population.
In many neural differentiation cultures, it can take a very long time for GFAP to be expressed (greater than 1 month). We have observed that GFAP expression can be augmented by culturing cells in media formulated for astrocyte growth (see below).
Cell debris can sometimes bleed into the neuronal cell population and use of live-dead discriminators can be useful for delineating the neuronal population.
Cell Populations: This kit has been developed for use on neural cell populations that have been derived from human pluripotent stem cells using the methods described in the figure legend below. This kit has not been tested on other cell types, including primary cells, and results may differ when examining cell types of a different origin (e.g higher background staining). In these instances, investigators may find it helpful to use isotype controls to distinguish non-specific background staining from specific antibody staining.
Warnings & Precautions
Danger: BD Phosflow™ Perm Buffer III (component 51-9006607) contains 87.68% methanol (w/w).
Hazard statements
Highly flammable liquid and vapor.
Toxic if swallowed, in contact with skin or if inhaled.
Causes damage to the central nervous system. Route of exposure: Oral.
Precautionary statements
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
Wear protective clothing / eye protection.
Wear protective gloves.
Do not breathe mist/vapours/spray.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower.
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Danger: Fixation Buffer (component 51-9006276) contains 4.2% formaldehyde (w/w).
Hazard statements
Harmful if inhaled.
Causes skin irritation.
Causes serious eye damage.
May cause an allergic skin reaction.
Suspected of causing genetic defects.
May cause cancer. Route of exposure: Inhalative.
May cause respiratory irritation.
Precautionary statements:
Wear protective clothing / eye protection.
Wear protective gloves.
Do not breathe mist/vapours/spray.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.
Continue rinsing.
If skin irritation or rash occurs: Get medical advice/attention.
Product Notices
- Please observe the following precautions: Absorption of visible light can significantly alter the energy transfer occurring in any tandem fluorochrome conjugate; therefore, we recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to prevent exposure of conjugated reagents, including cells stained with those reagents, to room illumination.
- Alexa Fluor® 488 fluorochrome emission is collected at the same instrument settings as for fluorescein isothiocyanate (FITC).
- Alexa Fluor® 647 fluorochrome emission is collected at the same instrument settings as for allophycocyanin (APC).
- Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- The Alexa Fluor®, Pacific Blue™, and Cascade Blue® dye antibody conjugates in this product are sold under license from Molecular Probes, Inc. for research use only, excluding use in combination with microarrays, or as analyte specific reagents. The Alexa Fluor® dyes (except for Alexa Fluor® 430), Pacific Blue™ dye, and Cascade Blue® dye are covered by pending and issued patents.
- PerCP-Cy5.5–labelled antibodies can be used with FITC- and R-PE–labelled reagents in single-laser flow cytometers with no significant spectral overlap of PerCP-Cy5.5, FITC, and R-PE fluorescence.
- PerCP-Cy5.5 is optimized for use with a single argon ion laser emitting 488-nm light. Because of the broad absorption spectrum of the tandem fluorochrome, extra care must be taken when using dual-laser cytometers, which may directly excite both PerCP and Cy5.5™. We recommend the use of cross-beam compensation during data acquisition or software compensation during data analysis.
- This PerCP-conjugated product is sold under license to the following patent: US Patent No. 4,876,190.
- Cy is a trademark of Amersham Biosciences Limited. This conjugated product is sold under license to the following patents: US Patent Nos. 5,486,616; 5,569,587; 5,569,766; 5,627,027.
- This product is subject to proprietary rights of Amersham Biosciences Corp. and Carnegie Mellon University and made and sold under license from Amersham Biosciences Corp. This product is licensed for sale only for research. It is not licensed for any other use. If you require a commercial license to use this product and do not have one return this material, unopened to BD Biosciences, 10975 Torreyana Rd, San Diego, CA 92121 and any money paid for the material will be refunded.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products


Development References (5)
-
Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009; 27(3):275-280. (Methodology: Cell differentiation). View Reference
-
Itsykson P, Ilouz N, Turetsky T, Goldstein RS et al. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol Cell Neurosci. 2005; 30(1):24-36. (Methodology: Cell differentiation). View Reference
-
Koch P, Opitz T, Steinbeck JA, Ladewig J, Brüstle O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci U S A. 2009; 106(9):3225-3230. (Biology). View Reference
-
Reubinoff BE, Itsykson P, Turetsky T, et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001; 19(12):1117-1118. (Methodology: Cell differentiation). View Reference
-
Yeo GW, Xu X, Liang TY, et al. Alternative splicing events identified in human embryonic stem cells and neural progenitors. PLoS Comput Biol. 2007; 3(10):1951-1967. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.