-
Your selected country is
Middle East / Africa
- Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?

Immunology
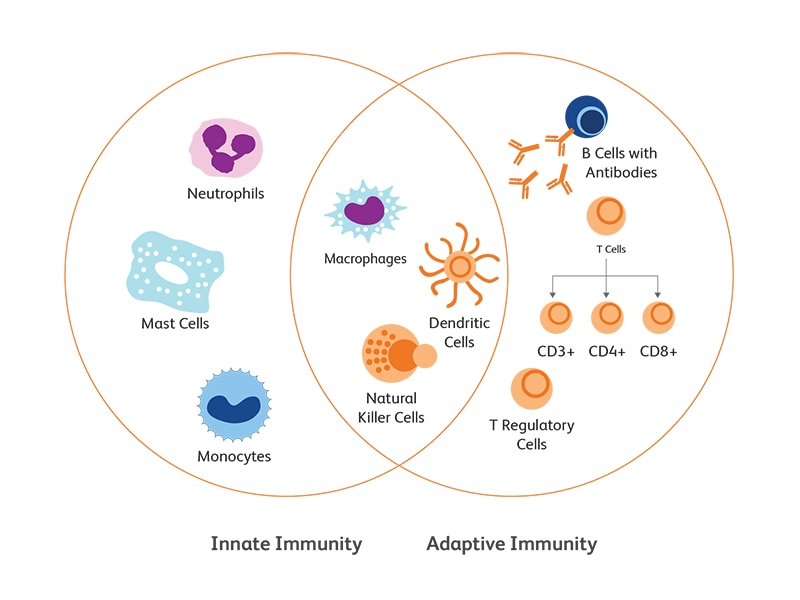
A healthy immune system functions through innate and adaptive immunity, the ability to defend against antigens, discriminate between self and nonself, remember previous infections, and cease to attack once the pathogen is removed from the system. The various types of immune cells, their intricate architecture and elaborate regulation, enable the body to maintain homeostasis and activate an adaptive immune response as needed. With flow cytometry, you are able to precisely and rapidly analyze a large number of immune cells, enumerate and characterize them, identify specific surface and intracellular markers of immune subtypes, and assess depletion of immune cells after procedures.
Innate versus adaptive immune responses
Innate immunity is the primary and immediate cellular response of the body to pathogenic attack through the recognition of conserved features of the pathogens. The recognition of molecular patterns associated with microbes is mediated by pattern-recognition receptors (PRRs), which include both surface receptors (Toll-like receptors (TLRs) and c-type lectin receptors (CLRs)) or cytoplasmic receptors (TLRs, nucleotide-binding oligomerization domain (Nod)-leucine-rich repeat-containing receptors (NLRs), RIG-I-like receptors (RLRs). PRRs recognize two types of ligands—conserved structures of microbial pathogens, known as PAMPs (pathogen associated molecular patterns), and danger signals from altered-self, known as DAMPs (danger associated molecular patterns). Activation of PRRs by their ligands gives rise to the mobilization of innate immune cells and the secretion of signaling and effector molecules (cytokines and antimicrobial peptides) leading to an inflammatory response to neutralize the pathogen or the danger.1 This first line of response is often a prerequisite to trigger initial responses from the adaptive immunity.
Adaptive immunity is elicited through IgGs (B cell receptors) on B lymphocytes (humoral) and T cell receptors (TCRs) on T lymphocytes (cell mediated). Somatic recombination through assembly of variable (V), diversity (D) and joining (J) gene segments enables amazing diversity of antigen recognition by Ig heavy chain and TCR β-chain genes.
Innate immune cells
Innate immune system comprises primarily of monocytes, macrophages, granulocytes, natural killer (NK) cells and dendritic cells (DCs).
Monocytes
Monocytes are the largest immune cells in the blood. They patrol the body and provide the first line of defense against bacterial and fungal intruders, launch inflammation via cytokine secretion and trigger adaptive immunity. Types of monocytes include classical or inflammatory monocytes characterized by CD14+ CD16− (humans), CCR2+ Ly6Chi (mice) and patrolling monocytes expressing CD14lo CD16+ (humans), CCR2lo Ly6Clo (mice).2
Macrophages
A hallmark of macrophages is their phagocytic capabilities supporting the clearance of intruders and priming immune responses. However, macrophages also enable other essential homeostatic functions such as metabolism.3 Macrophages are highly involved in tumor development and progression as tumor associated macrophages (TAMs) secrete factors inducing immune tolerance towards tumor cells. They are therefore of great interest in immuno-oncology and are central to investigations evaluating the impact of therapy on the tumor microenvironment (TME). Two main types of macrophages are described based on their stimuli induced polarization—M1 and M2. M1 macrophages are pro-inflammatory, promote an oxidative state with reactive oxygen species (ROS) production, and secrete inflammatory cytokines and chemokines, e.g., IL-1, IL-6, IL-12, TNF-α, CXCL9, CXCL10. M2 macrophages are considered immunosuppressive and secrete anti-inflammatory factors such as IL-10 and TGF-β.4
Granulocytes
Granulocytes are cells rich in cytoplasmic granules and include basophils5, neutrophils6,7, eosinophils8 and mast cells.9 Granulocytic cells support an array of protective functions including phagocytosis (neutrophils) defense against parasites (basophils) and as effectors in allergy (mast cells) and modulate host responses during immunotherapy.
Natural killer cell
Natural killer (NK) cells are generally categorized as innate immune cells, however they are similar to lymphocytes in their morphology and in the expression of lymphoid markers. Characteristic markers of NK cells include CD16, CD56 in human and NK1.1 in mice.10, 11
Dendritic cells
Dendritic cells (DCs), as professional antigen presenting cells, link innate and adaptive immunity by processing and presenting antigens to adaptive immune cells T and B lymphocytes. Three main types of dendritic cells are described:
- Plasmacytoid dendritic cells (pDCs) specialize in the recognition of viruses and tumor cells
- Type 1 classical dendritic cells (cDC1) recognize intracellular pathogens and trigger CD8 T cells and Th1 CD4 T cell responses
- Type 2 classical dendritic cells (cDC2) trigger CD4 T cell responses while in contact with intracellular pathogens, parasites, allergens, fungi and extracellular bacteria
Conventional markers of dendritic cells include CD11c, BDCA-1/2, CD123.12, 13
What is trained immunity?
Trained immunity or innate immune memory is the property of innate immune cells to adapt the host responses to mount resistance to past intruders.14 The response is still less specific than adaptive immune memory but can allow broad recognition of classes of pathogens such as Gram-positive and Gram-negative bacteria through the involvement of different types of pattern recognition receptors (PRRs).
Adaptive immune cells
The B and T lymphocytes are the main components of the adaptive immune arm and launch a delayed but more specific immune response to pathogens.15,16
T lymphocytes
T lymphocytes or T cells originate in the bone marrow and travel to the thymus for their maturation. Characteristic markers of T cells include CD3 and T cell receptor (TCR). They are later categorized by the expression of other surface molecules CD4 (CD4+ T cells) and CD8 (CD8+ T cells). Flow cytometry screening of surface and intracellular markers allows the distinction of the phenotype and functionality of subsets of T cells: Th1 (CD3, CD4, IFN-g, CCR5, CXCR3); Th2 (CD3, CD4, CCR3, CCR4, CXCR4, IL-4, IL-10); Th17 (CD3, CD4, CCR4, CCR6, TGFbRII, IL-17, CCL20); CD8+ cytotoxic T cells, T-reg or regulatory T cells (CD3, CD4, FoxP3, CD25, CTLA4, OX40/CD134, TGFb, IL-10, IL-35).17
B lymphocytes
B lymphocytes or B cells start their maturation in the bone marrow. B cells are known for their ability to support humoral immunity through the production of antibodies, but they carry other key functions such as phagocytosis and cross-presentation. As with other lymphocytes, markers of B cells evolve during their maturation and differentiation and include CD19, CD27 BCM, and CXCR4.18
The complement system
As innate and adaptive immune responses are mounted, they receive support from other body systems and processes including the complement system. The complement system is a network of membrane-bound and soluble protein factors that are engaged in three main pathways: classical complement pathway involving C-reactive protein (CRP), lectin pathway involving collections and mannose-binding lectin (MBL), and the alternative complement pathway. Activation of the complement cascade ultimately leads to cell lysis.19
Immunophenotyping of cells using flow cytometry
Flow cytometry is the key technique widely used to identify immune cell populations of interest based on types of surface markers and antigens. In addition to identifying lymphocyte subsets, the technique also enables isolation of rare cell types, detection of intracellular cytokines and quantitative analysis of cells. BD Biosciences offers several research cell sorters, cell analyzers and a comprehensive dye portfolio to support immunology research.
References
- Romo MR, Pérez-Martínez D, Ferrer CC. Innate immunity in vertebrates: an overview. Immunology. 2016;148(2):125-139. doi:10.1111/imm.12597
- Heung LJ. Monocytes and the host response to fungal pathogens. Front Cell Infect Microbiol. 2020;10:34. doi:10.3389/fcimb.2020.00034
- Mosser DM, Hamidzadeh K, Goncalves R. Macrophages and the maintenance of homeostasis [published online ahead of print, 2020 Sep 15]. Cell Mol Immunol. 2020;1-9. doi:10.1038/s41423-020-00541-3
- Petty AJ, Yang Y. Tumor-associated macrophages in hematologic malignancies: new insights and targeted therapies. Cells. 2019;8(12):1526. doi:10.3390/cells8121526
- Chirumbolo S, Bjørklund G, Sboarina A, Vella A. The role of basophils as innate immune regulatory cells in allergy and immunotherapy. Hum Vaccin Immunother. 2018;14(4):815-831. doi:10.1080/21645515.2017.1417711
- Liew PX, Kubes P. The neutrophil's role during health and disease. Physiol Rev. 2019;99(2):1223-1248. doi:10.1152/physrev.00012.2018
- Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P. Neutrophil diversity in health and disease. Trends Immunol. 2019;40(7):565-583. doi:10.1016/j.it.2019.04.012
- Simon HU, Yousefi S, Germic N, et al. The cellular functions of eosinophils: Collegium Internationale Allergologicum (CIA) Update 2020. Int Arch Allergy Immunol. 2020;181(1):11-23. doi:10.1159/000504847
- Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 2018;282(1):121-150. doi:10.1111/imr.12634
- Abel AM, Yang C, Thakar MS, Malarkannan S. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 2018;9:1869. doi:10.3389/fimmu.2018.01869
- Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44-49. doi:10.1126/science.1198687
- Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018;154(1):3-20. doi:10.1111/imm.12888
- Takenaka MC, Quintana FJ. Tolerogenic dendritic cells. Semin Immunopathol. 2017;39(2):113-120. doi:10.1007/s00281-016-0587-8
- Netea MG, Joosten LA, Latz E, et al. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. doi:10.1126/science.aaf1098
- Netea MG, Schlitzer A, Placek K, Joosten LAB, Schultze JL. Innate and adaptive immune memory: an evolutionary continuum in the host's response to pathogens. Cell Host Microbe. 2019;25(1):13-26. doi:10.1016/j.chom.2018.12.006
- Flajnik MF. A cold-blooded view of adaptive immunity. Nat Rev Immunol. 2018;18(7):438-453. doi:10.1038/s41577-018-0003-9
- Mousset CM, Hobo W, Woestenenk R, Preijers F, Dolstra H, van der Waart AB. Comprehensive phenotyping of T cells using flow cytometry. Cytometry A. 2019;95(6):647-654. doi:10.1002/cyto.a.23724
- Martínez-Riaño A, Bovolenta ER, Mendoza P, et al. Antigen phagocytosis by B cells is required for a potent humoral response. EMBO Rep. 2018;19(9):e46016. doi:10.15252/embr.201846016
- Lubbers R, van Essen MF, van Kooten C, Trouw LA. Production of complement components by cells of the immune system. Clin Exp Immunol. 2017;188(2):183-194. doi:10.1111/cei.12952
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.