Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ MiCK-1 Mouse Cytokine Positive Control Cells
(RUO)



Characteristic staining of MiCK-1 Positive Control Cells with IL-2, TNF, and IFN-γ. MiCK-1 cells were washed, permeabilized, and subsequently stained with PE-rat IgG1 isotype control (PE-R3-34; Cat. No. 554685; see Panel A), PE-rat anti-mouse IL-2 antibody (PE-JES6-5H4, Cat. No. 554428 see Panel B), PE-rat anti-mouse TNF (PE-MP6-XT22, Cat. No. 554419; Panel C), and PE-rat anti-mouse IFN-γ (PE-XMG1.2, Cat. No. 554412; Panel D). Despite fixation and freezing, the side- and forward-scattered light signals for these control cells (see Panel E) remain similar to those for freshly-prepared lymphoid cell preparations (data not shown). Bivariate markers for the gated monocyte population (Panel E) were set based on autofluorescence and isotype controls. The percentage of cytokine expressing cells was calculated based on the gated monocyte cell population shown in Panel E.


BD Pharmingen™ MiCK-1 Mouse Cytokine Positive Control Cells

MiCK-1 Mouse Cytokine Positive Control Cells
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Upon receipt, store the cell suspension at -80°C. Alternatively, the frozen cell suspension can be thawed and the capped vial should be quick spun to ensure complete retrieval of the contents from the vial. After thoroughly resuspending cells with a pipette, “single-use” aliquots can be refrozen and stored (-80°C) in polypropylene microtubes for a later time.
Recommended Assay Procedures
MiCK-1 Positive Control Cell suspensions are intended to provide cells that contain intracellular accumulations of IL-2, TNF, and IFN-γ which are detectable by immunofluorescent intracellular cytokine staining and flow cytometry. As such, these cells serve as positive controls for verifying the activity of fluorescent anti-cytokine antibodies and the staining procedure itself (e.g., permeabilization). For staining, the frozen cell preparation should first be quickly and carefully thawed. Aliquots of the cell suspension can then be transferred to microwells or tubes. The fixed and non-permeabilized cells should be washed twice with staining buffer to remove the dimethylsulfoxide. It is recommended that the washed cells first be stained with a fluorescent conjugate of RM4-5 (e.g., Cat. No. 553046, No. 553049, No. 553050, or No. 553051), an antibody which is known to bind to CD4 molecules expressed on the surface of fixed mouse CD4+ T cells. The cells must then be incubated for 10 min in permeabilization/wash buffer and washed. The cells are then stained with a fluorescent conjugate of either JES6-5H4 (rat anti-mouse IL-2 antibody; Cat. No. 554427, No. 554428, or No. 554429), MP6-XT22 (rat anti-mouse TNF; Cat. No. 554418, No. 554419, or No. 554420), or XMG1.2 (rat anti-mouse IFN-γ; Cat. No. 554411, No. 554412, or No. 554413).
Note: Cytokine-specific antibody staining of MiCK-1 cells can be demonstrated by preincubation of conjugated cytokine-specific antibody with recombinant cytokine or by pretreatment of the MiCK-1 cells with unlabled blocking antibody. The percentage of individual cytokine positive cells shown is representative of the product. Due to the cell activation procedure, a small proportion of cells may stain non-specifically (i.e., not blocked with either unlabeled antibody or ligand).
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Avoid contact with skin and eyes.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products
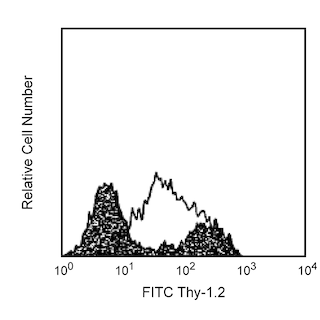

This suspension contains Mouse intracellular CytoKine-1 (MiCK-1) Positive Control Cells. The MiCK-1 cell suspension contains ~5 x 10^6 fixed, non-permeabilized mouse lymphoid cells. The suspension includes cells that express easily detectable levels of intracellular IL-2, TNF (also known as TNF-α), and IFN-γ as determined by immunofluorescent intracellular cytokine staining and flow cytometry. MiCK-1 cell suspensions were prepared by stimulating mouse spleen cells in the presence of a protein transport inhibitor. After stimulation, the cells were harvested and were incubated with Fc Block™ [rat IgG2b,κ anti-mouse CD16/CD32 (FcγII/III receptor) antibody; Cat. No. 553142] to reduce Fc receptor-mediated background staining. The cells were fixed and then stored in 10% dimethylsulfoxide and 90% fetal bovine serum at -80°C. Each lot of MiCK-1 contains a measurable proportion of cytokine-producing cells. Representative flow cytometric results are shown for typical staining of MiCK-1 cells. Data from individual lots of MiCK-1 cells may vary. Investigators should anticipate similar (though not identical) results to those shown due to differences in staining methodology and in flow cytometers/cytometer settings.
Development References (2)
-
BD Biosciences. Techniques for Immune Function Analysis, Application Handbook 1st Edition. 2003. Available: http://www.bdbiosciences.com/pdfs/manuals/02-8100055-21A1rr.pdf 2007, Jan. 25.
-
Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995; 188(1):117-128. (Methodology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.