Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?

Immune Monitoring
Clinical research utilizes the practice of monitoring the immune system to evaluate responses and changes in health and disease prior to or during therapy. This monitoring of the immune system supports several areas of immunology research, such as immuno-oncology, allergy and autoimmunity, and also includes monitoring responses to pathogenic infections, such as HIV, COVID-19 and others. With the increased awareness of antitumor immunity in mediating responses to immunotherapy approaches, the need to efficiently monitor immune responses has been steadily increasing. BD Biosciences provides several tools for clinical research on active immune monitoring.
Applications of immune monitoring
Monitoring tolerance in transplantation research
Immune monitoring is heavily used in clinical research seeking to understand the immune mechanisms driving tolerance in transplantation therapies under investigation. For example, in liver transplantation trials, passive tolerance such as spontaneous operational tolerance (SOT) can be monitored in the peripheral blood using markers of regulatory T cells, gamma delta T cells or NK cells. In kidney transplantation trials, it can be monitored using transitional and IL10+ granzyme B+ regulatory B cells.1
Immuno-oncology and evaluation of immunotherapy
During immunotherapy research protocols, immune monitoring allows the evaluation of the reactivity of immune responses at the populational and single-cell level. Major immunotherapy strategies, including CAR T cell therapy, transplantation and immune checkpoint inhibitors, can all benefit from immune monitoring tools before, during and post-intervention to inform on the status of the procedure and also understand the molecular signatures to develop stratification strategies.2,3
Immune monitoring for precision medicine trials
Immune monitoring tools are also used in the clinical research that is required prior to clinical trials of biologic/biosimilars in order to assess long-term safety and patient-specific therapeutic strategies to support precision medicine initiatives.4 In asthma with a TH2 endotype, monitoring of immune biomarkers such as periostin and elevated eosinophilis direct the orientation of therapy towards TH2 response components. Immune monitoring applied in the studies of allergy could support strategies to prevent disease development and to determine theoretical thresholds to further study patient-specific dose escalation schedules to allergens.
Immune monitoring in allergy research
Allergic reactions involve different populations of immune cells, including antigen presenting cells (e.g., dendritic cells), mast cells, Ig-E producing B cells and T cells. Different types of allergic hypersensitivity exist based on the types of immune cells involved (e.g., IgG, IgM or antigen-specific T cells) and cytokines released (e.g., IL-4, IL5). Allergic reactions can also be non-IgG mediated. Measuring serum cytokines, complement activation or mitochondrial function are some strategies for measuring allergic responses in immune monitoring research.5,6
Immune monitoring for surveillance in immuno-oncology
The tumor microenvironment (TME) plays a critical role in the maintenance of the tumor and its response to therapy. The TME is composed of the tumor itself, its surroundings and all interacting cells and cellular processes the tumor can re-route to survive. These include cancer stem cells, infiltrating immune cells, blood vessels that carry nutrients, signaling molecules as well as the extracellular matrix (ECM) that allows migration of cancer cells to other sites.7
Immune monitoring in metabolism studies
Immune monitoring helps in understanding tumor metabolism and the metabolic share of the immunosuppressive TME. Nutrient access allows the tumor to gain essential replenishment to thrive and modulate key metabolic pathways (e.g., cholesterol synthesis, mitochondrial energy production, glucose metabolism) to help direct TME immune cell functions. Competition between tumor cells and T cells for the same nutrients leads to metabolic reprogramming of immune cell functions in the TME. Metabolic therapies (e.g., COX inhibitors for lipid metabolism, mTOR inhibitors for glucose metabolism) modify the nutrients made available to the TME and have been shown to reprogram tumor infiltrating T cells and re-boost antitumor immunity. Immune monitoring of tumor infiltrating immune cells gives access to the phenotype and functionality of these cells and informs on the immune activities in the TME, all affecting tumor burden and response to therapy.8
Immune monitoring in autoimmunity research
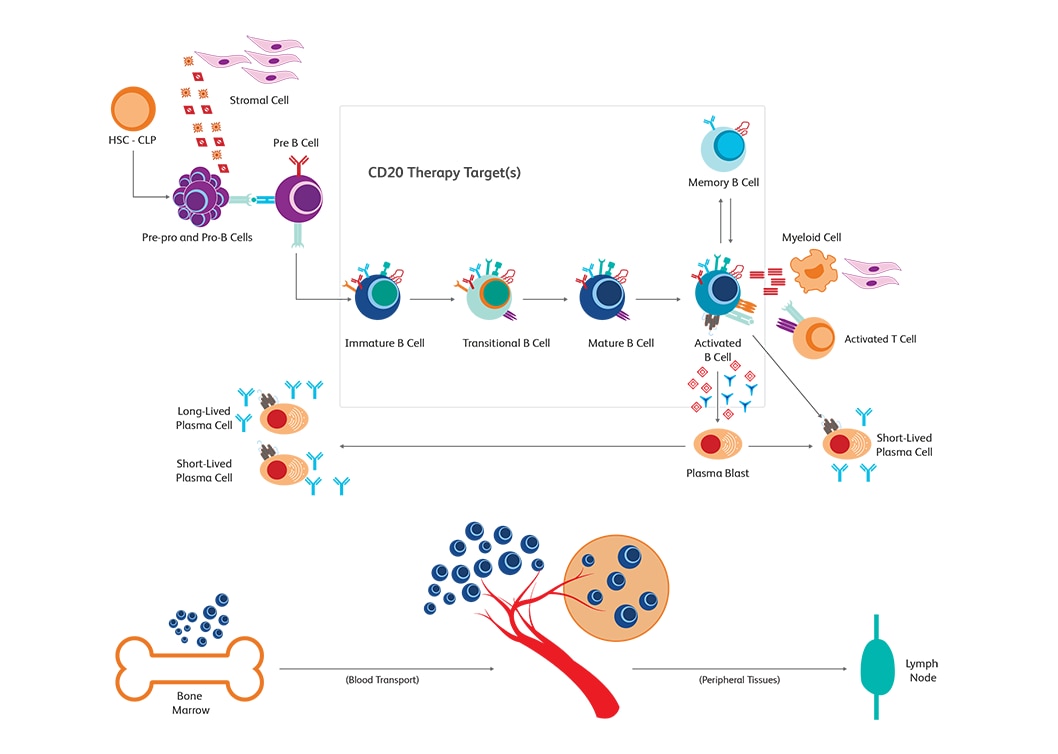
When the immune system fails to distinguish self from non-self and elicits responses that are typically meant for defending the host from antigens, autoimmune disorders ensue. With an increased understanding of the role of B cells in autoimmune disease pathogenesis, targeting B cells has also emerged as an alternative method for tackling autoimmune diseases. Memory and effector B cells could be targeted to prevent generation of pathogenic antibodies and subsequently block the synthesis of cytokines. B cells have been used increasingly in autoimmunity research.9
What are the challenges in immune monitoring?
Archival versus fresh samples
Immune monitoring of immune cell markers can be challenging depending on the material being examined and its preparation. For example, cryopreservation can alter the conformation of surface proteins and make them more difficult to detect by immunostaining; therefore, some experiments are preferentially conducted on fresh materials as it is more representative of the live condition.10 Sample preparation methods can also affect the sample yield and the quality of subsequent experiments. BD Biosciences offers various solutions to collect, process and isolate specimens. The BD Horizon™ Dri Tumor & Tissue Dissociation Reagent (TTDR) offers gentle and effective dissociation with good epitope preservation. TTDR maximizes cell yields, while minimizing cell death, which allows effective dissociation of a variety of tumor types to enable single-cell studies.
Status of markers to be assessed
In translational immuno-oncology research, assessment of paired samples provides a comparative approach when studying therapy-induced immune responses, evaluating static markers at initial diagnosis and dynamic markers throughout the course of treatment. Any immune monitoring tool should be able to assess the exact status of these markers, which can be challenging.
Material or sample abundance
The abundance and viability of the sample plays a critical role in experimental design and may limit the scope of experimentation. Multicolor flow cytometry enables assessment of multiple markers at the same time, leveraging the full potential of the sample. Designing the panel appropriately helps enormously in effectively utilizing precious samples. BD Biosciences offers several panel design tools and resources to make this process easier.
Tools for immune monitoring research
Flow cytometry
Cell surface markers are regularly used for monitoring the heterogeneity of subsets of cells.11,12,13
Immunohistochemistry
Immunohistochemistry (IHC) is an immunostaining technique and as such uses the ability of specific antibodies to detect antigens of interest in tissue samples. Immunohistochemistry is routinely used in several research areas and in clinical research for a variety of conditions. Associated with microscopy, it allows the visualization of stained immune cells in fixed or live biospecimens.
Single-cell multiomics
Single-cell multiomics allows in-depth analyses of various applications, including immunology, oncology and metabolism. BD Biosciences provides single-cell multiomics tools, such as the BD Rhapsody™ Single-Cell Analysis System and BD® AbSeq Assays, which combine RNA and protein biomarker expression and powerful bioinformatics tools, such as FlowJo™ v10 Software and SeqGeq™ v1.6 Software, to help you obtain multiomics data and unlock useful insights from them.
Assays for immune monitoring research
BD offers a comprehensive portfolio of reagents and kits to study immune cells from different perspectives, using a range of technologies. Among the methods used for immune monitoring, the preeminent method is multicolor flow cytometry because it enables the characterization of highly complex subpopulations—both functionally and phenotypically. Combined with other complementary technologies such as ELISA, ELISPOT and BD® Cytometric Bead Array, information unavailable from multicolor flow, such as levels of secreted molecules, can be captured.
The BD Horizon™ Dri TBNK + CD20 Multicolor Panel is optimized for immune monitoring research. This tube has been used in research and clinical trials, from monitoring immune responses to CD20 depletion therapies. The panel characterizes T, B and NK cells. The Dri format eliminates manual pipetting, increasing efficiency and provides absolute counts when paired with BD Trucount™ Tubes.
The 7-color BD Horizon™ Treg Panel is optimized for cell therapy research and immune system research. The tube contains a dried, pre-aliquoted multicolor cocktail comprising markers used for the characterization of FoxP3+ naïve, transitional and effector Treg subsets. Applications for this panel in research and clinical research trials include but not limited to autoimmunity studies, transplant studies, tumor microenvironment studies and infection.
References
- Sani KB, Sawitzki B. Immune monitoring as prerequisite for transplantation tolerance trials. Clin Exp Immunol. 2017;189(2):158-170. doi:10.1111/cei.12988
- Hartmann FJ, Babdor J, Gherardini PF, et al. Comprehensive immune monitoring of clinical trials to advance human immunotherapy. Cell Rep. 2019;28(3):819-831.e4. doi:10.1016/j.celrep.2019.06.049
- Przespolewski A, Szeles A, Wang ES. Advances in immunotherapy for acute myeloid leukemia. Future Oncol. 2018;14(10):963-978. doi: 10.2217/fon-2017-0459
- Seyhan A, Carini C. Are innovation and new technologies in precision medicine paving a new era in patients centric care? J Transl Med. 2019;17(1):114. doi: 10.1186/s12967-019-1864-9
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16(12):751-765. doi:10.1038/nri.2016.111
- Simon D, Cianferoni A, Spergel JM, et al. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Allergy. 2016;71(5):611-620. doi:10.1111/all.12846
- Arneth B. Tumor microenvironment. Medicina (Kaunas). 2019;56(1):15. doi: 10.3390/medicina56010015
- Cuyàs E, Verdura S, Martin-Castillo B, et al. Tumor cell-intrinsic immunometabolism and precision nutrition in cancer immunotherapy. Cancers (Basel). 2020;12(7):1757. doi:10.3390/cancers12071757
- Du FH, Mills EA and Mao-Draayer Y. Next-generation anti-CD20 monoclonal antibodies in autoimmune disease treatment. Autoimmunity Highlights. 2017;8:12. doi: 10.1007/s13317-017-0100-y
- Wargo JA, Reddy SM, Reuben A, Sharma P. Monitoring immune responses in the tumor microenvironment. Curr Opin Immunol. 2016;41:23-31. doi:10.1016/j.coi.2016.05.006
- Stojanovic I, Ruivo CF, van der Velden TJG, Schasfoort RBM, Terstappen LWMM. Multiplex label free characterization of cancer cell lines using surface plasmon resonance imaging. Biosensors (Basel). 2019;9(2):70. doi: 10.3390/bios9020070
- Sukhdeo K, Paramban RI, Vidal JG, et al. Multiplex flow cytometry barcoding and antibody arrays identify surface antigen profiles of primary and metastatic colon cancer cell line. PLOS One. 2013;8(1):e53015. doi: 10.1371/journal.pone.0053015
- Greve B, Kelsch R, Spaniol K, Eich HT, Götte M. Flow cytometry in cancer stem cell analysis and separation. Cytometry A. 2012;81(4):284-293. doi: 10.1002/cyto.a.22022
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.