Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Hoechst 33342 Solution
(RUO)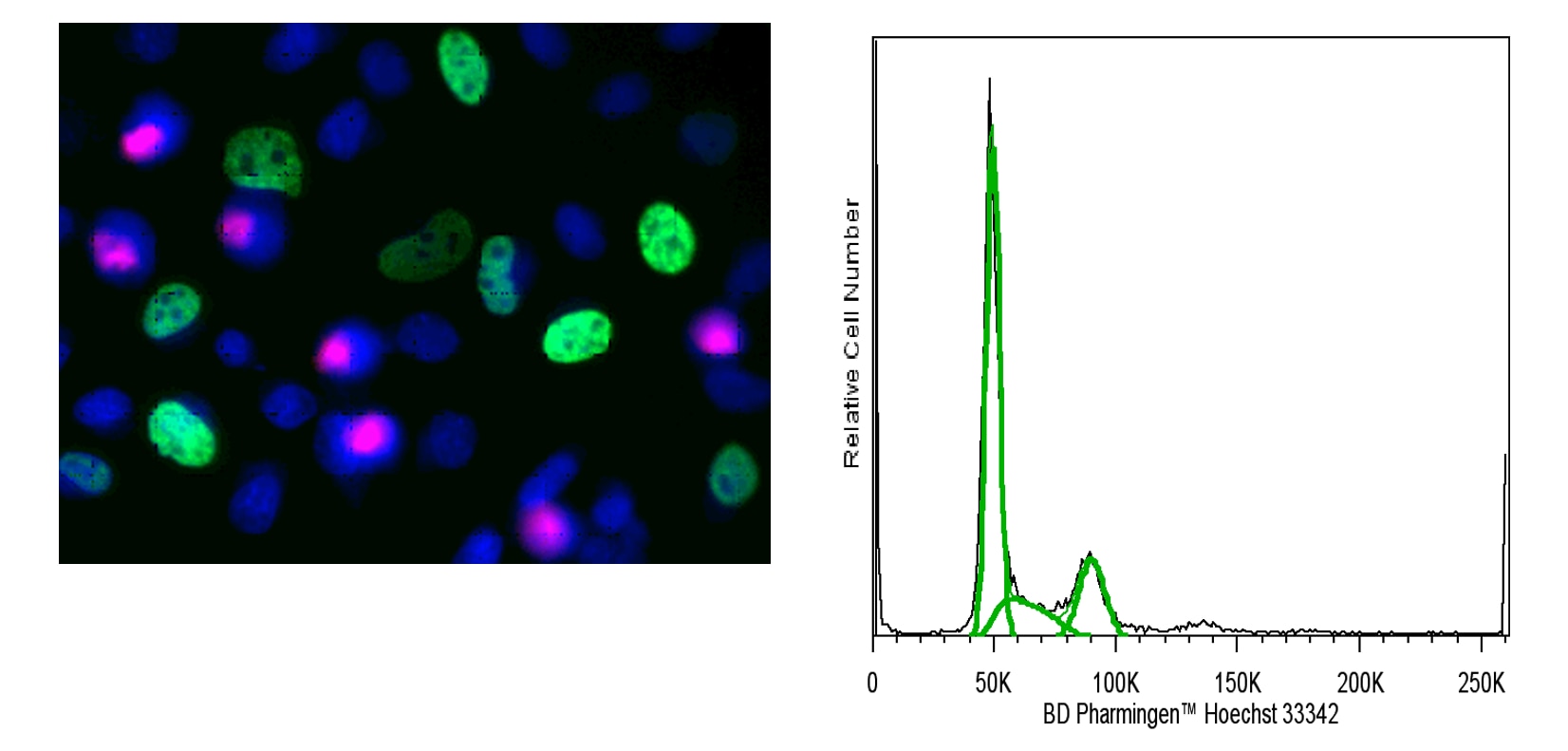



Multicolor immunofluorescence analysis of cell cycle of HeLa cells (Left Panel). HeLa cells (ATCC CCL-2) were stained with BD Pharmingen™ Cell Cycle Kit (Cat. No. 558662) with Alexa Fluor® 488 Mouse anti-BrdU ( pseudo-colored green), Alexa Fluor® 647 Rat anti-Histone H3 (pS28) (pseudo-colored red), and Hoechst 33342 (Cat. pseudo-colored blue). Co-staining of Hoechst 33342 and Histone H3 (pS28) appears pink. The confocal image was captured using a 20x (0.75 NA) objective with the BD Pathway™ 435 Bioimaging Analyzer and merged using BD Attovision™ Software. Flow cytometric analysis of HeLa cell DNA content (Right panel). HeLa cells in log growth were dissociated from the growth medium using Cell Dissociation Buffer (Life Technologies) and resuspended in complete medium containing 10 μg/mL Hoechst 33342 for 60 minutes at 37°C. Cells were pelleted by centrifugation, Hoechst-containing medium was removed, and cells were resuspend in PBS and analyzed for DNA content. Data was acquired on a BD LSRFortessa™ cell analyzer system. DNA content histogram was deconvoluted into G0/G1, S, and G2/M populations by FlowJo software (TreeStar).


BD Pharmingen™ Hoechst 33342 Solution

Hoechst 33342 Solution
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
The BD Pharmingen™ Hoechst 33342 Solution is a reagent for the fluorescent staining of DNA and nuclei in live or fixed cells. Hoechst 33342 is a bisbenzimidazole dye with high specificity for binding to double-stranded DNA (preferentially binds to A-T base pairs). This dye is very useful to label double-stranded DNA and thus to visualize nuclei. Hoechst 33342 can be excited at ~355 nm by a UV light source (eg, UV laser beam or a mercury arc-lamp). It emits blue fluorescence light around an emission maximum at 461 nm when bound to DNA. Since the Hoechst 33342 dye is specific for DNA binding, ribonuclease treatment is not needed to avoid nonspecific RNA staining. In addition to its use in fluorescence microscopy and image analysis, Hoechst 33342 is commonly used for flow cytometric applications, such as cell cycle analysis and stem cell side population identification.
Preparation And Storage
Recommended Assay Procedures
Immunofluorescent Staining of Live Cells for Nuclear Visualization
1. Dilute Hoechst 33342 solution to 5 - 10 μg/mL in complete medium immediately prior to use.
2. Add Hoechst 33342 solution to each sample and incubate at 37°C for 30 - 60 minutes. The stain time required is cell type dependent.
3. Remove Hoechst solution from cells at the end of the incubation period and add BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) or 1× PBS. Cells may also be analyzed without washing, but this may increase background from unbound dye.
4. Proceed to imaging.
Immunofluorescent Staining of Fixed Cells for Nuclear Visualization
1. Fix and permeabilize cells as desired.
2. Dilute Hoechst 33342 solution to 2 µg/ml in 1× PBS immediately prior to use.
3. Add 2ug/ml Hoechst 33342 solution to each sample at least 15 minutes before analysis.
4. Proceed to imaging.
Staining of Live Cells for DNA Content Analysis by Flow Cytometry
1. Obtain a single cell suspension.
2. Resuspend cells at 1x10^6 cells/mL or less in complete medium containing 5 - 10 μg/mL Hoechst 33342.
Note: Alternatively, Hoechst 33342 may be added directly to culture medium without pelleting if the culture cell density does not exceed 1x10^6 cells/mL.
3. Incubate at 37°C for 30 - 60 minutes.
a. The optimal cell density, concentration of Hoechst 33342, and stain time for DNA content analysis may vary by cell type. Assay conditions should be optimized in early experiments for best results.
4. Pellet cells by centrifugation and aspirate medium containing Hoechst 33342.
5. Resuspend cells in BD Pharmingen™ Stain Buffer (FBS) or 1× PBS and proceed to analysis by flow cytometry.
Staining of Fixed Cells for DNA Content Analysis by Flow Cytometry
1. Obtain a single cell suspension.
2. Treat cells on ice for 30 minutes with 70 - 80% ice-cold ethanol.
a. Ethanol fixation typically provides the most resolved histograms. However, this reagent has also been successfully used for DNA content analysis with the Transcription Factor Buffer Set (Cat. No. 562574) or BD Cytofix™ Fixation Buffer (Cat. No. 554655) and BD Phosflow™ Perm III (Cat. No. 558050) protocol.
3. Wash cells once with BD Pharmingen™ Stain Buffer (FBS).
4. Dilute Hoechst 33342 solution to 1 - 5 μg/mL in BD Pharmingen™ Stain Buffer (FBS) or 1× PBS immediately prior to use.
5. Stain cells for 15 minutes at a cell density of 1x10^6 cells/mL. No wash is necessary prior to analysis.
a. The optimal cell density and concentration of Hoechst 33342 for DNA content analysis may vary by cell type. Assay conditions should be optimized in early experiments for best results.
6. Proceed to analysis by flow cytometry.
This product is also available as a component of the Cell Cycle Kit (Cat. No. 558662). Please see the kit's Technical Data Sheet for a detailed protocol for the use of Hoechst Dye 333342 in conjunction with immunofluorescent staining of plated cells.
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- This antibody has been developed and certified for the bioimaging application. However, a routine bioimaging test is not performed on every lot. Researchers are encouraged to titrate the reagent for optimal performance.
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- FlowJo is a trademark of Tree Star Inc.
Companion Products


Development References (3)
-
Crissman HA, Steinkamp JA. Multivariate cell analysis. Techniques for correlated measurements of DNA and other cellular constituents. In: Gray JW, Darzynkiewicz Z, ed. Techniques in Cell Cycle Analysis. Clifton, NJ: Humana Press; 1987:163-206.
-
Müller W, Gautier F. Interactions of heteroaromatic compounds with nucleic acids. Eur J Biochem. 1975; 54(2):385-394. (Methodology). View Reference
-
Shapiro HM. Flow cytometric estimation of DNA and RNA content in intact cells stained with Hoechst 33342 and pyronin Y. Cytometry. 1981; 2(3):143-150. (Methodology: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.