Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
The BD FastImmune™ CD107a APC reagent is designed for the detection of degranulating T lymphocytes in activated whole blood and is intended for research use only. Applications include studies of cytotoxic CD8+ T-cell responses to viral and tumor antigens, correlation of T-cell cytolytic potential and cytokine expression, and live-cell sorting of functional antigen-specific CD8+ T cells. The CD107a detection assay can also be used as an alternative to 51Cr release assays.
Each vial supplies sufficient reagents for 50 stimulated samples and 50 unstimulated control samples. In performing the assay, 0.5 mL of whole blood is stimulated with antigen (not included) in the presence of two secretion inhibitors, monensin and brefeldin A (BFA), and CD107a antibody. For the unstimulated control, 0.5 mL of blood is treated with the secretion inhibitors and CD107a, but not antigen. Both blood samples are then stained for IFN-γ or other cytokine(s), as well as the gating markers CD3 and CD8 (not included).
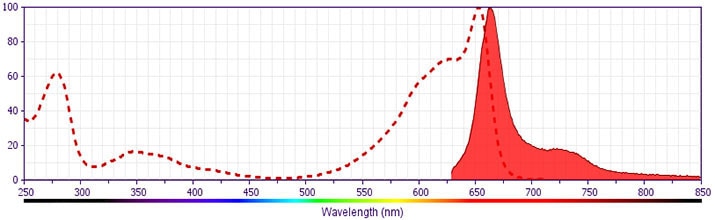
Development References (11)
-
Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Meth. 2003; 281:65-78. (Biology).
-
Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004; 75:487-512. (Biology).
-
Betts MR, Price DA, Brenchley JM, et al. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J Immunol. 2004; 172:6407-6417. (Biology).
-
Centers for Disease Control. Perspectives in disease prevention and health promotion update: universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in health-care settings. MMWR. 1988; 37:377-388. (Biology).
-
Clinical and Laboratory Standards Institute. 2005. (Biology).
-
Jones N, Eggena M, Baker C, et al. Presence of distinct subsets of cytolytic CD8+ T cells in chronic HIV infection. AIDS Res Hum Retroviruses. 2006; 22:1007-1013. (Biology).
-
Laird DW, Castillo M, Kasprzak L. Gap junction turnover, intracellular trafficking, and phosphorylation of connexin43 in brefeldin A-treated rat mammary tumor cells. J Cell Biol. 1995; 131(5):1193-1203. (Biology). View Reference
-
Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Evaluation of the CD107 cytotoxicity assay for the detection of cytolytic CD8+ cells recognizing HER2/neu vaccine peptides. Breast Cancer Res Treat. 2005; 92:85-93. (Biology).
-
Nagorsen D, Scheibenbogen C, Thiel E, Keilholz U. Immunological monitoring of cancer vaccine therapy. Expert Opin Biol Ther. 2004; 4:1677-1684. (Biology).
-
Peters PJ, Borst J, Oorschot V, et al. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991; 173:1099-1109. (Biology).
-
Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003; 9:1377-1382. (Biology).
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Refer to manufacturer's instructions for use and related User Manuals and Technical Data Sheets before using this product as described.
Comparisons, where applicable, are made against older BD technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.