Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?

Clinical Areas
For Professionals in Clinical Diagnostics
Flow cytometry has been an invaluable tool in several clinical applications. BD Biosciences offers a comprehensive portfolio of clinical flow cytometers and reagents and kits that can be used for diagnostic by health care professionals purposes in various clinical applications.
Blood Cancers
Blood cancers involve disruptions of the hematopoietic and immune systems, leading to a variety of hematologic malignancies. Leukemia, lymphoma (Hodgkin and non-Hodgkin) and myeloma are the three major categories of blood cancer. Flow cytometry immunophenotyping is one diagnostic step in blood cancers. We offer a wide array of tools, including clinical cell analyzers and single-color monoclonal antibodies with many color options; assays may be for in vitro diagnostic use (IVD), whilst others may include our range of Analyte Specific Reagents (ASR) as building blocks* for laboratory developed tests for immunophenotyping and analysis.
Transfusion
Enumerating residual leukocytes in leukoreduced blood products is critical to ensure the quality of blood components. Flow cytometry is an effective counting method for evaluating leukoreduced products. BD Biosciences offers clinical flow cytometers and assays for enumerating residual leukocytes in leukoreduced blood products.
Transplantation
For many hematopoietic malignancies, collection and infusion of CD34+ hematopoietic stem/progenitor cells following chemotherapy is critical. An accurate measurement of CD34 is important for dose requirement protocols in stem cell transplantation. We offer clinical flow cytometers and assays, such as the BD® Stem Cell Enumeration (SCE) Kit, for enumerating CD34+ cells.
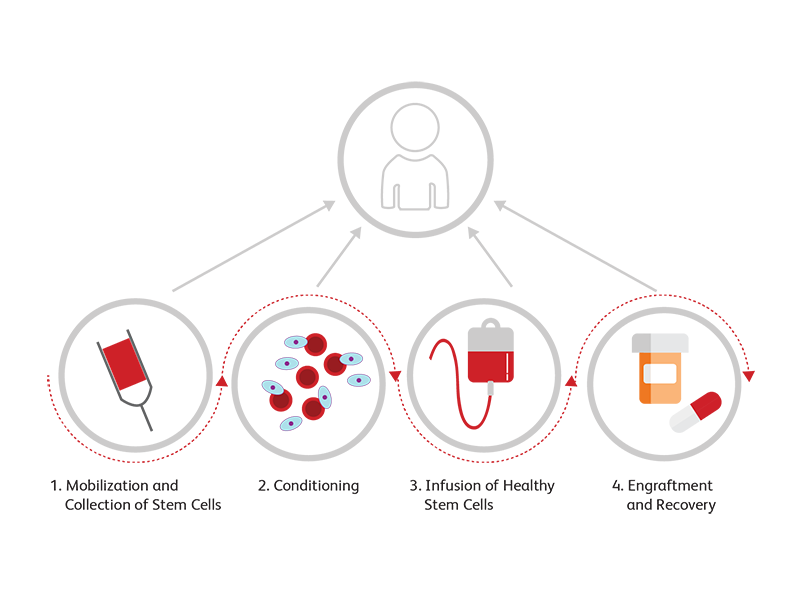
Immune Deficiencies
Alterations in the innate or adaptive arms of the immune system can lead to immune deficiencies. The most prominent secondary immune infection, acquired immune deficiency syndrome (AIDS), results from infection with human immunodeficiency virus (HIV). HIV specifically infects and reduces the number of CD4+ subsets of T lymphocytes. BD Biosciences offers robust clinical solutions for assessment of lymphocyte populations in persons living with HIV.
Rheumatology
In autoimmune disorders the immune system overresponds against one’s own antigens. This failure to distinguish between self from nonself arises from breach of immune tolerance, the preventative mechanisms the immune system has in place to prevent attacking itself. Autoimmune diseases, such as ankylosing spondylitis, are caused by dysfunction in immune responses. BD Biosciences provides a tool for detecting HLA-B27, which is used to screen for ankylosing spondylitis, a rheumatic disorder. Our clinical BD® HLA-B27 Kit offers rapid detection of HLA-B27 antigen expression in erythrocyte-lysed whole blood using the BD FACSCalibur™ and BD FACSCanto™ Flow Cytometers.
HLA-B27 test is not for use in tissue typing.
The BD® Stem Cell Enumeration (SCE) Kit provides simultaneous enumeration of viable dual-positive CD45+/CD34+ hematopoietic stem cell populations in CD34+ absolute counts (cells/μL) as well as the percentage of the total viable leucocyte count that is CD34+ (%CD34). The following specimens can be analyzed with this kit: normal and mobilized peripheral blood, fresh and thawed leucopheresis products, fresh and thawed bone marrow, and fresh and thawed cord blood. The kit is intended for in vitro diagnostic (IVD) use on either the BD FACSCalibur™ Flow Cytometer using BD CellQuest™ or BD CellQuest™ Pro Software or the BD FACSCanto™ II Flow Cytometer using BD FACSCanto™ Clinical Software.
BD Flow Cytometers are Class 1 Laser Products.
Unless otherwise noted, BD products described in this page are For In Vitro Diagnostics Use.
*BD Biosciences clinical flow cytometry solutions, including instrumentation, software and reagents, offer the building blocks for laboratory-developed tests for immunophenotyping and analysis of blood cancers. These solutions are not FDA cleared or approved for the diagnosis of blood cancers. Analyte Specific Reagent. Analytical and performance characteristics are not established.
Refer to manufacturer's instructions for use and related User Manuals and Technical Data Sheets before using this product as described.
Comparisons, where applicable, are made against older BD technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.