Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?



Reimagine How You Work
BD® Research Cloud is a unique ecosystem for flow cytometry experiments to drive visibility, teamwork and efficiency for your lab.

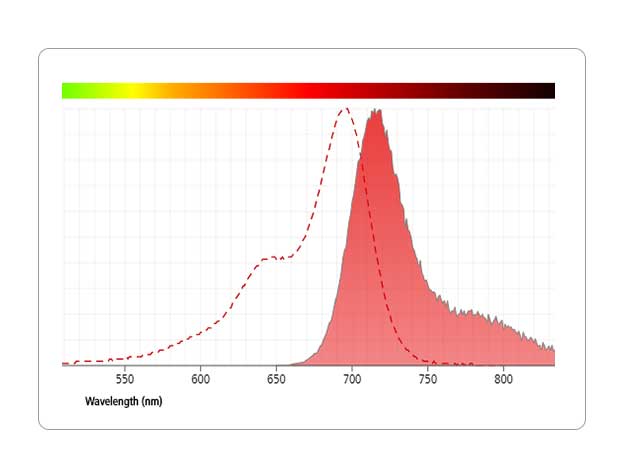
Breakthrough with Blue
Minimize spillover and maximize breakthroughs with laser-specific BD Horizon RealBlueTM 780 Reagents.
BD Horizon™ Human T Cell Backbone Panel
Expand your research with a pre-optimized and flexible panel, designed to help you expand your research with confidence.
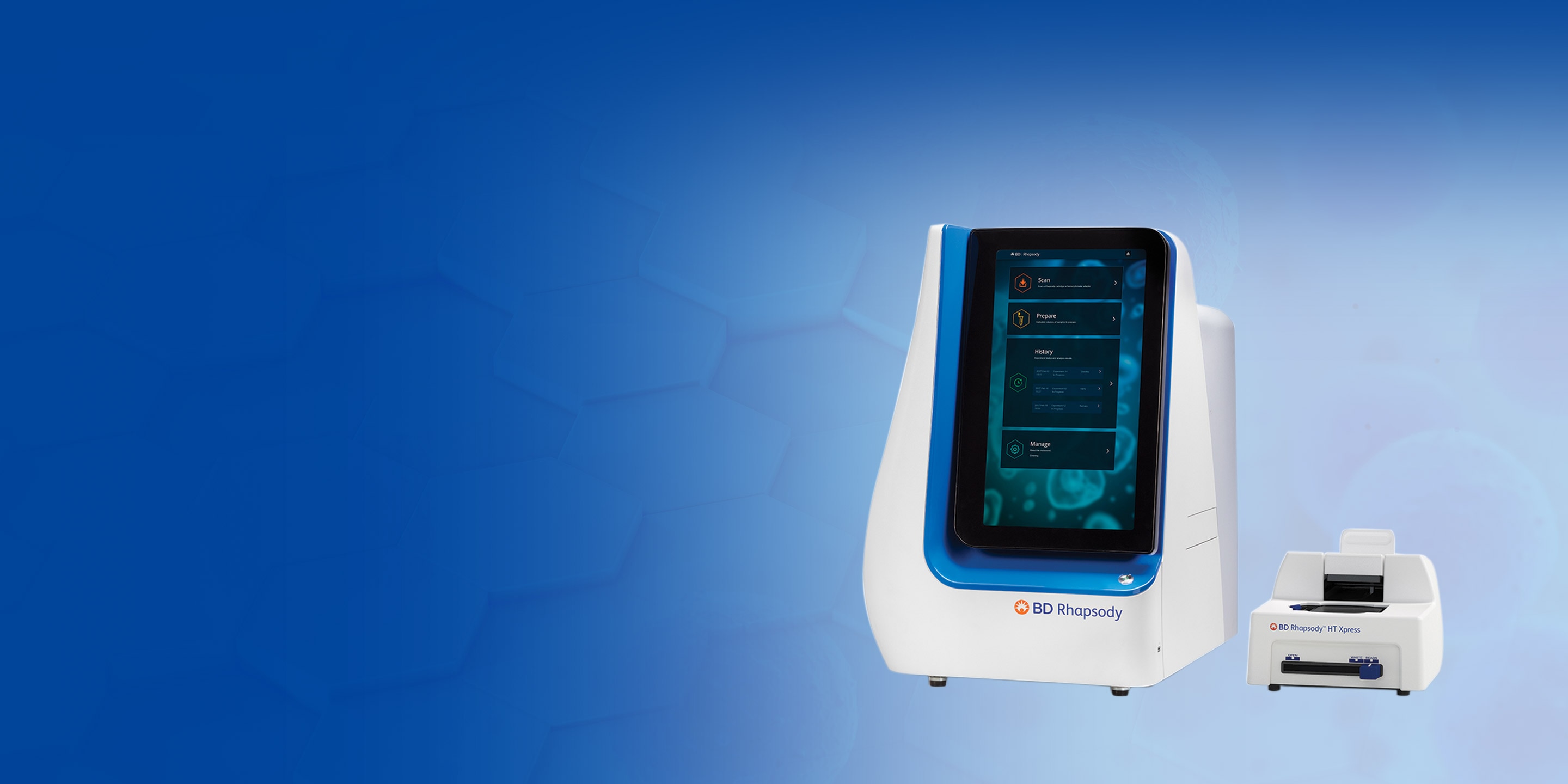
Introducing BD Rhapsody™ HT Single-Cell Analysis System
Fast track single-cell research without compromise

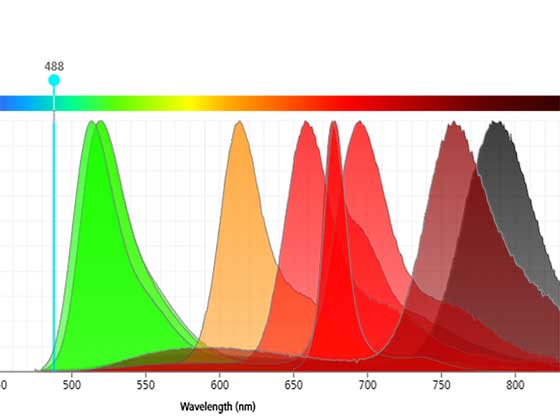
Visualize the Future of Flow Cytometry
Derive greater insights and unlock new discoveries using image-enabled spectral sorting.
Catch the Live Flow
BD CellView™ Image Technology amplifies your flow data with spatial and morphological insights


Applications and Solutions
A comprehensive suite of trusted products, integrated solutions, useful tools and a wealth of information to advance your flow cytometry applications.
Innovative solutions to power your diagnostics and research
Backed by cutting-edge technology and more than 45 years of flow cytometry expertise
Utilize our featured resources with invaluable information to advance your science.
Explore a variety of tools for your multicolor flow cytometry, from panel design to selection tools—an array of aids to support your research.
Be part of the flow cytometry community with the latest flow cytometry news, thought leader opinions, blogs on breakthrough research, interesting flow cytometry publication reviews, and more.
Use our excellent product and technical support with our >45 years of flow cytometry expertise and support resources.
Find a seamless customer experience and an easy way to create and manage accounts.
Explore a variety of ways that work with your purchasing process to place and track orders.
Explore our webinar series spanning a broad range of topics and in-depth reviews by senior scientists, professionals and clinicians.
Use a variety of training courses for instruments and applications to take full advantage of the capabilities of BD products.
Refer to manufacturer's instructions for use and related User Manuals and Technical Data Sheets before using this product as described.
Comparisons, where applicable, are made against older BD technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.