Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
![V450 Mouse Anti-Human IFN-α[2b]](/content/dam/bdb/products/global/reagents/flow-cytometry-reagents/research-reagents/single-color-antibodies-ruo/561xxx/5613xx/561382_base/561382Image1.png)
![V450 Mouse Anti-Human IFN-α[2b]](/content/dam/bdb/products/global/reagents/flow-cytometry-reagents/research-reagents/single-color-antibodies-ruo/561xxx/5613xx/561382_base/561382Image1.png)

![V450 Mouse Anti-Human IFN-α[2b]](/content/dam/bdb/products/global/reagents/flow-cytometry-reagents/research-reagents/single-color-antibodies-ruo/561xxx/5613xx/561382_base/561382Image1.png)
Multicolor flow cytometric analysis of IFN-alpha2b in stimulated peripheral blood mononuclear cells (PBMC). Human PBMC were stimulated using CpG oligodeoxynucleotide (Coley Pharmaceutical Co., Cat. No. 2336) and then treated with BD FastImmune™ Brefeldin A (BFA) Solution (Cat. No. 347688). Cells were harvested, washed, and stained with FITC-conjugated Lin-1 Cocktail (Cat. No. 340546), PerCP-Cy™5.5 Mouse Anti-Human CD123 (Cat. No. 558714/560904), and APC Mouse Anti-Human HLA-DR (Cat. No. 559868) simultaneously. The cells were then fixed and permeabilized (see Recommended Assay Procedure) followed by intracellular staining with BD Horizon™ V450 Mouse Anti-Human IFN-α2b (IFN-alpha2b; Cat. No. 561382). The two-color flow cytometric dot plots show the expression of CD123 versus IFN-alpha (Right Panel) derived from gated events with the light scattering characteristics of lymphocytes and monocytes and the immunofluorescence characteristics of Lin-1 negative and HLA-DR positive cells (Left Panel). Flow cytometry was performed using a BD™ LSR II Flow Cytometer System.

![V450 Mouse Anti-Human IFN-α[2b]](/content/dam/bdb/products/global/reagents/flow-cytometry-reagents/research-reagents/single-color-antibodies-ruo/561xxx/5613xx/561382_base/561382Image1.png)
BD Horizon™ V450 Mouse Anti-Human IFN-α[2b]

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
PROCEDURE
Cell Activation
Note: The following cellular activation procedure needs to be performed in a tissue culture hood using aseptic technique.
1. Isolate fresh human peripheral blood mononuclear cells (PBMC) from 60 ml of fresh blood, and wash twice with sterile 1X Dulbecco's Phosphate Buffered Saline (PBS). Centrifuge the cells at 250g for 10 minutes and discard the supernatant.
2. Suspend the cells in complete tissue culture medium (RPMI supplemented with 1% Pen/Strep, 1% L-glutamine and 10% fetal bovine serum). Count and adjust the cell concentration to 2 to 3 million cells/mL.
3. Label two sterile 50-mL conical tubes as "Not Stimulated" and "Stimulated with CpG." Dispense PBMC to each tube (2 million cells/test)
4. Add 5 µg of CpG oligodeoxynucleotide (Coley Pharmaceutical Co. Cat. No. 2336) per ml of cells to the "Stimulated with CpG" tube. Cap the tube and vortex gently.
5. Incubate both tubes for 2 hours at 37°C.
6. Dilute BD FastImmune™ Brefeldin A (BFA) Solution (Cat. No. 347688) 1 to 10 in sterile 1X PBS.
7. Add 20 µl of 1X BFA per mL to both tubes ("Not Stimulated" and "Stimulated with CpG"). Incubate tubes at 37°C for 2 hours.
Note: Keep both CPG and BFA aliquots at -20°C.
8. Add 100 µl of 20 mM EDTA (Sigma Cat. No. E7889) per mL of cells to both tubes and incubate overnight at 4°C.
Surface and Intracellular Staining
9. Centrifuge the two tubes at 250g for 10 minutes. Discard the supernatants by aspiration and resuspend cells in 25 mL of BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656).
10. Centrifuge the tubes at 250g for 10 minutes. Discard the supernatants and resuspend cells in Stain Buffer (FBS) at 20 million per ml.
11. Label 12 × 75 mm polypropylene tubes appropriately. Add 100 µl of cells to each tube.
12. Add surface staining antibodies to each tube: Human Lin-1 FITC (Cat. No. 340546), Human CD123 PerCPCy5.5 (Cat. No. 558714/560904), and Human HLA-DR APC (Cat. No. 559868). Incubate the tubes at room temperature for 30 minutes in the dark.
13. Following incubation, add 2 mL of cold Stain Buffer (FBS) to each tube and centrifuge at 250g for 10 minutes. Discard the supernatants by aspiration and vortex the pellets to resuspend the cells.
14. Add 1 mL of room temperature BD Cytofix/Cytoperm Buffer (Cat. No. 554722) to each tube. Mix well and incubate at room temperature in the dark for 30 minutes.
15. Add 2 mL of cold BD Perm/Wash™ Buffer (Cat. No. 554723) to each tube and centrifuge at 500g for 5 minutes; discard the supernatants by aspiration and vortex the pellets to resuspend cells. Repeat the wash step again.
16. Add the fluorescent intracellular staining antibody, BD Horizon™ V450 Mouse Anti-Human IFN-α2b (IFN-alpha2b; Cat. No. 561382; 5 µl/test) or the proper fluorescent immunoglobulin isotype control at the appropriate volume per test to the tubes and mix well by vortexing. Bring test volume to 100 µl using cold BD Perm/Wash Buffer. Incubate tubes at room temperature for 60 minutes in the dark.
17. Add 2 mL of cold BD Perm/Wash Buffer to each tube and centrifuge at 500g for 5 minutes; discard supernatant by aspiration and vortex pellet to suspend cells.
18. Add 500 µl of cold Stain Buffer to each tube for immediate flow cytometric analysis.
Optional: Resuspend the cell pellets with 200 µl of cold 1% -formaldehyde solution and keep the tubes at 4°C in the dark up to 24 hours before flow cytometry. If storing longer than 24 hours, we recommend washing cells in Stain Buffer (FBS) as extended incubation with fixatives might affect fluorochromes.
Flow Cytometry and Data Analysis
Acquire at least 500,000 events (lymphocytes and monocytes).
A sequential gating strategy is required for successful data analysis:
1. Gate tightly on the lymphocytes and monocytes using the forward- and side-light scatter profiles.
2. View the Lin-1 versus HLA-DR profile of the gated lymphocytes and monocytes, and select the Lin-1-negative, HLA-DR-positive population. Be sure not to include any of the Lin-1-positive cells.
3. View the IFN-alpha2b (IFN-alpha) versus CD123 profile of the gated cells to detect the IFN-alpha-positive cell population.
The "Not Stimulated" sample is used as a negative control that can be used to set markers for measuring the frequency of IFN-α2b-positive (IFN-alpha-positive) cells.
Open up the second gate on Lin-1- HLA-DR+ cells and from there open up the third gate for CD123+ IFN-alpha+ cells. Acquire about 150 to 200 events in the CD123+ IFN-alpha+ double positive cell quadrant.
Product Notices
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- BD Horizon V450 has a maximum absorption of 406 nm and maximum emission of 450 nm. Before staining with this reagent, please confirm that your flow cytometer is capable of exciting the fluorochrome and discriminating the resulting fluorescence.
- Pacific Blue™ is a trademark of Molecular Probes, Inc., Eugene, OR.
Companion Products



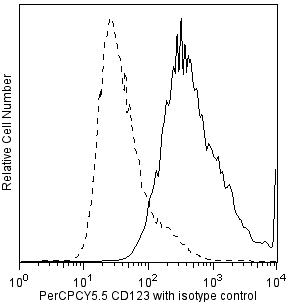

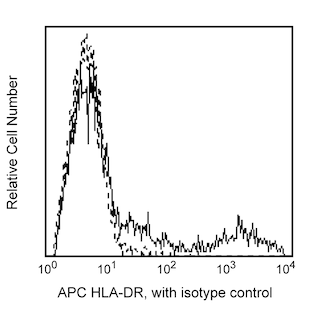
The 7N4-1 antibody reacts with human IFN-α2b and to a lesser extent with IFN-α7. It does not react with IFN-α1 nor IFN-α4. IFN-α2b is one of the three variants of IFN-α2 that have been isolated from human cell lines. IFN-α2b is the variant predominantly produced by human leukocytes. Human IFN-α2b belongs to the IFN-α class of proteins also known as leukocyte interferons. IFN-α comprises a family of related but distinct proteins with molecular weights ranging from 16-27 kDa with antiviral, antiproliferative and immunomodulatory activities. The IFN-α family is composed from as many as 14 different genes. The immunogen used to generate the 7N4-1 hybridoma was E. coli-expressed recombinant human IFN-α2b. This is a neutralizing antibody.
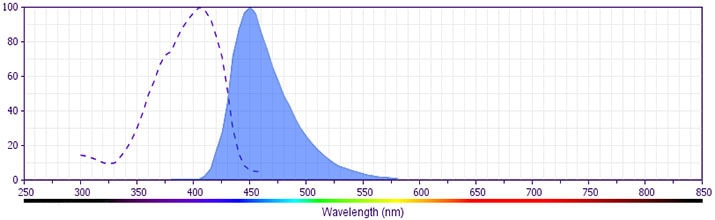
The antibody is conjugated to BD Horizon™ V450, which has been developed for use in multicolor flow cytometry experiments and is available exclusively from BD Biosciences. It is excited by the Violet laser Ex max of 406 nm and has an Em Max at 450 nm. Conjugates with BD Horizon™ V450 can be used in place of Pacific Blue™ conjugates.
Development References (7)
-
Dipaola M, Smith T, Ferencz-Biro K, Liao MJ, Testa D.. Interferon-alpha 2 produced by normal human leukocytes is predominantly interferon-alpha 2b. J Interferon Res. 1994; 14(6):325-332. (Biology). View Reference
-
Henco K, Brosius J, Fujisawa A, et al. Structural relationship of human interferon alpha genes and pseudogenes. J Mol Biol. 1985; 185(2):227-260. (Biology). View Reference
-
Liao MJ, Lee N, Dipaola M, et al. Distribution of interferon-alpha 2 genes in humans. J Interferon Res. 1994; 14(4):183-185. (Biology). View Reference
-
Lydon NB, Favre C, Bove S, et al. Immunochemical mapping of alpha-2 interferon. Biochemistry. 1985; 24(15):4131-4141. (Immunogen). View Reference
-
Pestka S. The human interferons—from protein purification and sequence to cloning and expression in bacteria: before, between, and beyond. Arch Biochem Biophys. 1983; 221(1):1-37. (Biology). View Reference
-
Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999; 284(5421):1835-1837. (Clone-specific: Immunocytochemistry (cytospins)). View Reference
-
Vogel, S., R. Friedman, M. Hogan. J.E. Coligan, A.M. Kruisbeek, D.H. Margulies, E.M. Shevach and W. Strober, ed. Current Protocols in Immunology. New York: John Wiley & Sons; :1-920.
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.