Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Horizon™ Fixable Viability Stain 780
(RUO)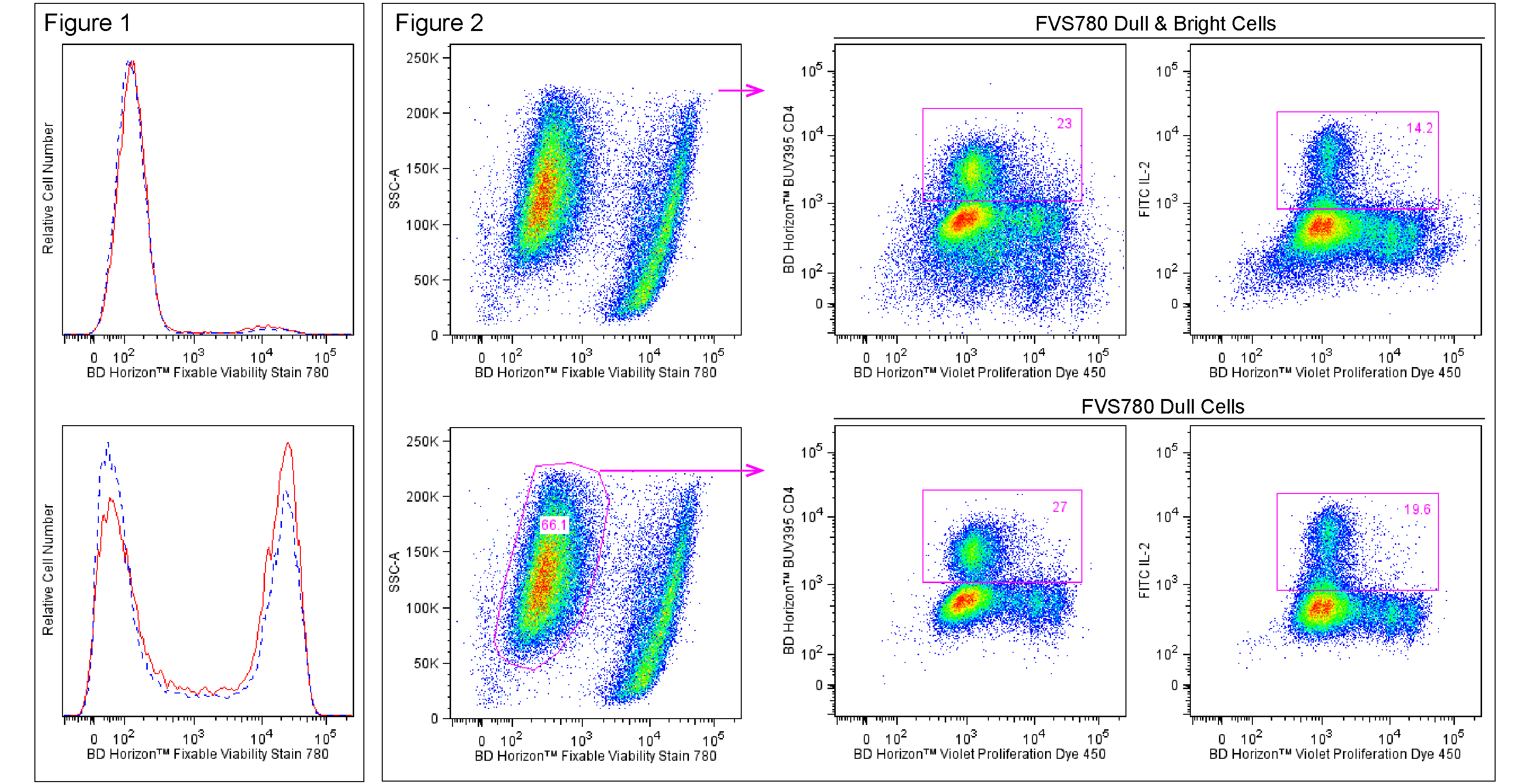


Figure 1. Fluorescent staining of Jurkat cells with BD Horizon™ Fixable Viability Stain 780. Human Jurkat cells were treated (16 hr) with 0.025% DMSO (Top Plot) or 5 μM camptothecin (Bottom Plot) and stained with BD Horizon™ Fixable Viability Stain 780 (Cat. No. 565388). Cells were either not fixed (solid line histograms), or fixed in BD Cytofix™ Fixation Buffer (Cat. No. 554655) and permeabilized in Perm/Wash Buffer I (Cat. No. 557885) (dashed line histograms). Histograms were derived from gated events with the light scattering characteristics of Jurkat cells. Flow cytometry was performed using a BD™ LSRII Cell Analyzer System. Figure 2. Analysis of proliferating mouse splenocytes for surface and intracellular markers. BALB/c splenocytes were stained (10 min, 37°C) with BD Horizon™ Violet Proliferation Dye 450 (Cat. No. 562158), washed twice, and then cultured (3 days) with Purified NA/LE Hamster Anti-Mouse CD3e (Cat. No. 553057) and Hamster Anti-Mouse CD28 (Cat. No. 553294) antibodies. The cells were restimulated (4 hr) with PMA, Ionomycin, and BD GolgiStop™ Protein Transport Inhibitor (Monensin) (Cat. No. 554724). Cells were harvested, stained with BD Horizon™ Fixable Viability Stain 780, fixed and permeabilized using a BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit (Cat. No. 554714), and then stained with BD Horizon™ BUV395 Anti-Mouse CD4 (Cat. No. 563790) and FITC Anti-Mouse IL-2 (Cat. No. 554427) antibodies. Two-color dot plots showing VPD450 fluorescence versus CD4 expression (Middle Plots) or IL-2 expression (Right Plots) were derived from either total cells (Top; FVS780 Dull and Bright Cells) or previously viable cells (Bottom; FVS780 Dull Cells) with the light scatter characteristics of intact cells. CD4+ and IL-2+ cell gates were based on an FMO or unstimulated cell control, respectively. Flow cytometry was performed on a BD LSRFortessa™ Cell Analyzer system.

BD Horizon™ Fixable Viability Stain 780
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Recommended Assay Procedures
Preparation
Bring FVS780 dye powder and 180 μl of fresh cell culture-grade Dimethyl Sulfoxide (DMSO; eg, Sigma D2650) to room temperature. Add 180 μl of DMSO and vortex solution well. Inspect the solution and repeat vortex until the stock dye has fully dissolved. This is the Stock Solution.
Storage
Upon arrival, store the dry dye desiccated and protected from light at -80°C until use. After reconstitution with DMSO, store the Stock Solution at -20°C in small aliquots. Do not use reconstituted dye after 90 days of storage. Please discard the dye solution after 90 days post reconstitution with DMSO.
Cytometry Requirements
Red laser-equipped Flow Cytometers (eg, BD FACSCanto™ II, BD™ LSR II, BD LSRFortessa™, or BD Accuri™ C6) can be used. This dye can be read out of filters commonly used for APC-Cy™7 (eg, 780/60). Fluorescence compensation is best achieved using cell samples of interest. When designing multicolor staining panels, please be aware of fluorescence spillover into the BD Horizon™ BUV737, BD Horizon™ BV786, and PE-Cy™7 (when read off the yellow-green laser) channels. Panels should be optimized to take this spillover into account. To reduce fluorescence spillover, we recommend titrating FVS780 and using the lowest possible dye concentration that provides adequate resolution of live and dead cell populations of interest.
Procedure
Fixable Viability Stain 780 labeling of cells
1. Prepare cells for flow cytometric staining using sodium azide-free buffers.
2. Wash cells one time in sodium azide- and protein-free Dulbecco's Phosphate Buffered Saline (1X DPBS).
3. Resuspend cells at 1-10x10^6 cells/ml in sodium azide- and protein-free 1X DPBS.
4. Add 1 μl of BD Horizon™ Fixable Viability Stain 780 Stock Solution for each 1 ml of cell suspension (1:1000) and vortex immediately.
a. Note: We recommend titrating the dye for optimal performance, as different cell types and different applications can result in a wide degree of variability in staining.
5. Incubate the mixture for 10-15 minutes at room temperature protected from light.
a. Optional: Alternatively, incubate mixtures at 37°C for 5-7 minutes or 2-8°C for 30-60 minutes.
6. Wash cells twice with 2 ml of BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) or the equivalent.
7. Decant the supernatant and gently mix to disrupt the cell pellet.
8. Resuspend the cells in Stain Buffer (FBS) or equivalent.
9. Stain, fix and permeabilize cells as desired for downstream applications.
Notes:
1. Each user should determine the optimal concentrations of reagents, cells, and conditions for the assay of interest. We recommend titrating the reagent in early experiments to obtain optimal results.
2. The reactivity of the free dye is quenched by washing with buffer containing protein (eg, FBS or BSA).
3. Cells may be stained in bulk prior to freezing or staining with fluorescent antibodies.
4. BD Horizon™ Fixable Viability Stain 780 can be used in intracellular staining assays that require fixation with formaldehyde and permeabilization with methanol and detergents such as those used for BD Phosflow™ staining (eg, Cat. No. 558050, BD Phosflow™ Perm Buffer III), intracellular cytokine staining (eg, Cat. No. 554714, BD Cytofix/Cytoperm™ Fixation/Permeabilization Kit), or transcription factor staining (eg, Cat. No. 562574/562725, BD Pharmingen™ Transcription Factor Buffer Set).
5. Apoptotic cells can show variable staining. We recommend co-staining with, eg, Annexin V FITC (Cat. No. 556419) if further analysis is desired for the apoptotic cells.
Danger: Causes serious eye damage.
Precuationary statements: Wear protective gloves/protective clothing/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
Immediately call a POISON CONTROL/doctor.
Dispose of contents/containers in an appropriate treatment and disposal facility in accordance with applicable laws and regulations, and product characteristics at time of disposal.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Cy is a trademark of GE Healthcare.
- Before staining with this reagent, please confirm that your flow cytometer is capable of exciting the fluorochrome and discriminating the resulting fluorescence.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products





BD Horizon™ Fixable Viability Stain 780 (FVS780) is useful for discrimination of viable from non-viable mammalian cells in multicolor flow cytometric applications. This dye reacts with and covalently binds to cell-surface and intracellular amines. Permeable plasma cell membranes, such as those present in necrotic cells, allow for the intracellular diffusion of the dye and covalent binding to higher overall concentrations of amines than in non-permeable live cells. Therefore, necrotic cells present in a typical in vitro assay label with higher levels of dye increasing their fluorescence intensity 10-20 fold over that of viable cells. The labeled cells can be fixed with formaldehyde for downstream decontamination, freezing and/or permeabilization and subsequent intracellular staining while maintaining stable viability stain fluorescence.
BD Horizon™ Fixable Viability Stain 780 is excited by the Red laser (with an excitation maximum of 759 nm), and has a fluorescence emission maximum of 780 nm.
Development References (2)
-
Perfetto SP, Chattopadhyay PK, Lamoreaux L, et al. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J Immunol Methods. 2006; 313(1–2):199-208. (Methodology). View Reference
-
Perfetto SP, Chattopadhyay PK, Lamoreaux L, et al. Amine-reactive dyes for dead cell discrimination in fixed samples. Curr Protoc Cytom. 9(9.34)(Methodology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.