Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD IMag™ Human NK Cell Enrichment Set - DM
(RUO)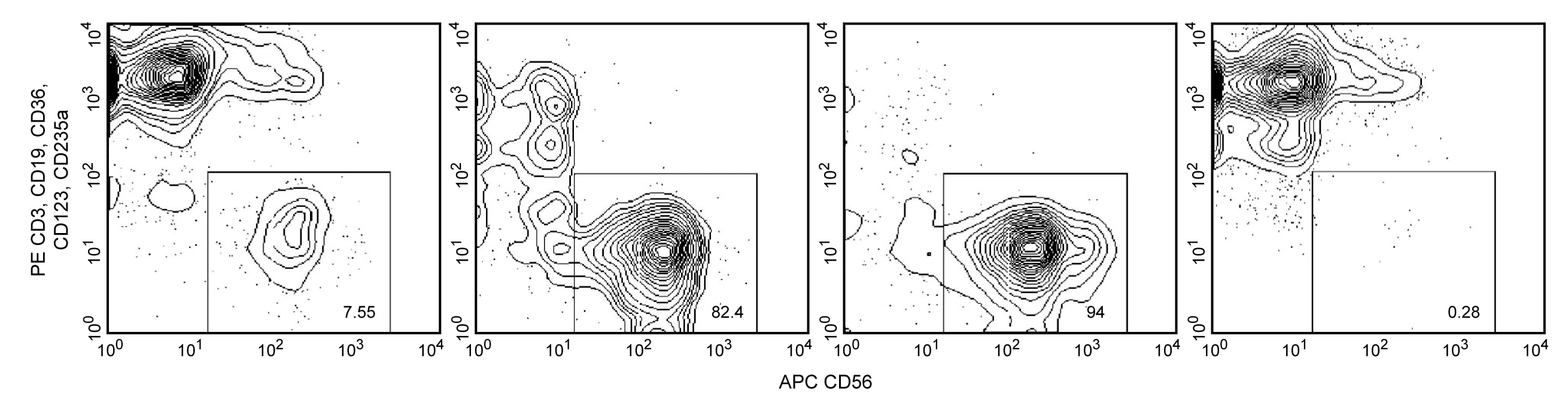


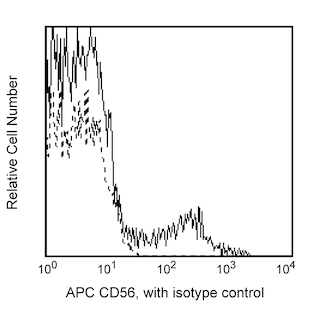
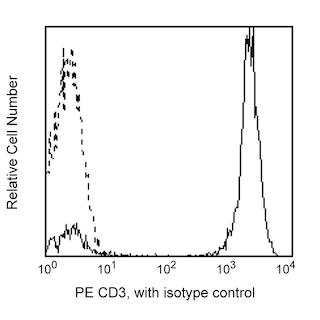
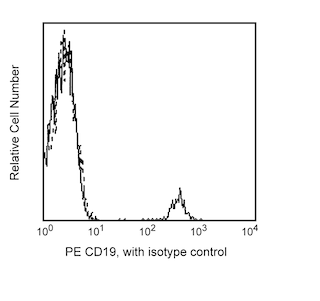
Enrichment of NK cells from human blood. PBMC were labeled with the BD IMag™ Human NK Cell Enrichment Set - DM (Cat. No. 557987) and separated on the BD™ IMag™ Cell Separation Magnet (Cat. No. 552311) according to the accompanying protocol. To demonstrate the efficiency of the enrichment, cells were stained with APC Anti-Human CD56 (Cat. No. 555518) to detect NK cells and a mixture of PE Mouse Anti-Human CD3 (Cat. No. 555333), Anti-Human CD19 (Cat. No. 555413), Anti-Human CD36 (Cat. No. 555455), Anti-Human CD123 (Cat. No. 555644), and Anti-Human CD235a (Cat. No. 555570) monoclonal antibodies to detect non-NK leukocytes (including NK-T cells) and erythrocytes. Dead cells were excluded by staining with Propidium Iodide Staining Solution (Cat. No. 556463), and leukocytes were selected by scatter profile. Flow cytometry was performed on a BD FACSCalibur™ flow cytometry system. Please refer to the Enrichment Flow Chart to identify the cell populations represented in this figure. The percentage of NK cells is indicated in the lower-right corner of each panel. Far left panel shows unseparated PBMC. Middle left panel shows the combined enriched fraction after three 6-minute magnetic separations. Middle right panel shows the twice-enriched fraction after an additional 6-minute separation of the cells shown in middle left panel. This additional separation step typically results in >5% increased purity with less than a 5% decrease in recovery. Far right panel shows the positive fraction.



BD IMag™ Human NK Cell Enrichment Set - DM

BD IMag™ Human NK Cell Enrichment Set - DM

Human NK Cell Enrichment Set - DM
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
The BD IMag™ Human NK Cell Enrichment Set - DM is used for the negative selection of Natural Killer (NK) cells from peripheral blood. The Biotinylated Human NK Cell Enrichment Cocktail contains monoclonal antibodies that recognize antigens expressed on erythrocytes, platelets, and peripheral leukocytes (including NK-T cells) that are not NK cells. The BD IMag™ Streptavidin Particles Plus - DM are magnetic nanoparticles that have streptavidin covalently conjugated to their surfaces. With these two components, the BD IMag™ Human NK Cell Enrichment Set -DM avoids the inadvertent activation of the enriched NK cells by using reagents that do not directly bind to those NK cells. This Enrichment Set has been optimized for use with the BD IMag™ Cell Separation Magnet, and it contains sufficient reagents to label 10^9 peripheral blood mononuclear cells (PBMC).
The NK Cell Enrichment Cocktail component is comprised of the following biotin-conjugated monoclonal antibodies:
Anti-human CD3, clone UCHT1
Anti-human CD19, clone HIB19
Anti-human CD36, clone CB38 (NL07)
Anti-human CD41a, clone HIP8
Anti-human CD66b, clone G10F5
Anti-human CD123 (IL-3 Receptor α chain), clone 9F5
Anti-human CD235a (Glycophorin A), clone GA-R2 (HIR2)
Anti-human IgE, clone G7-26
Preparation And Storage
Recommended Assay Procedures
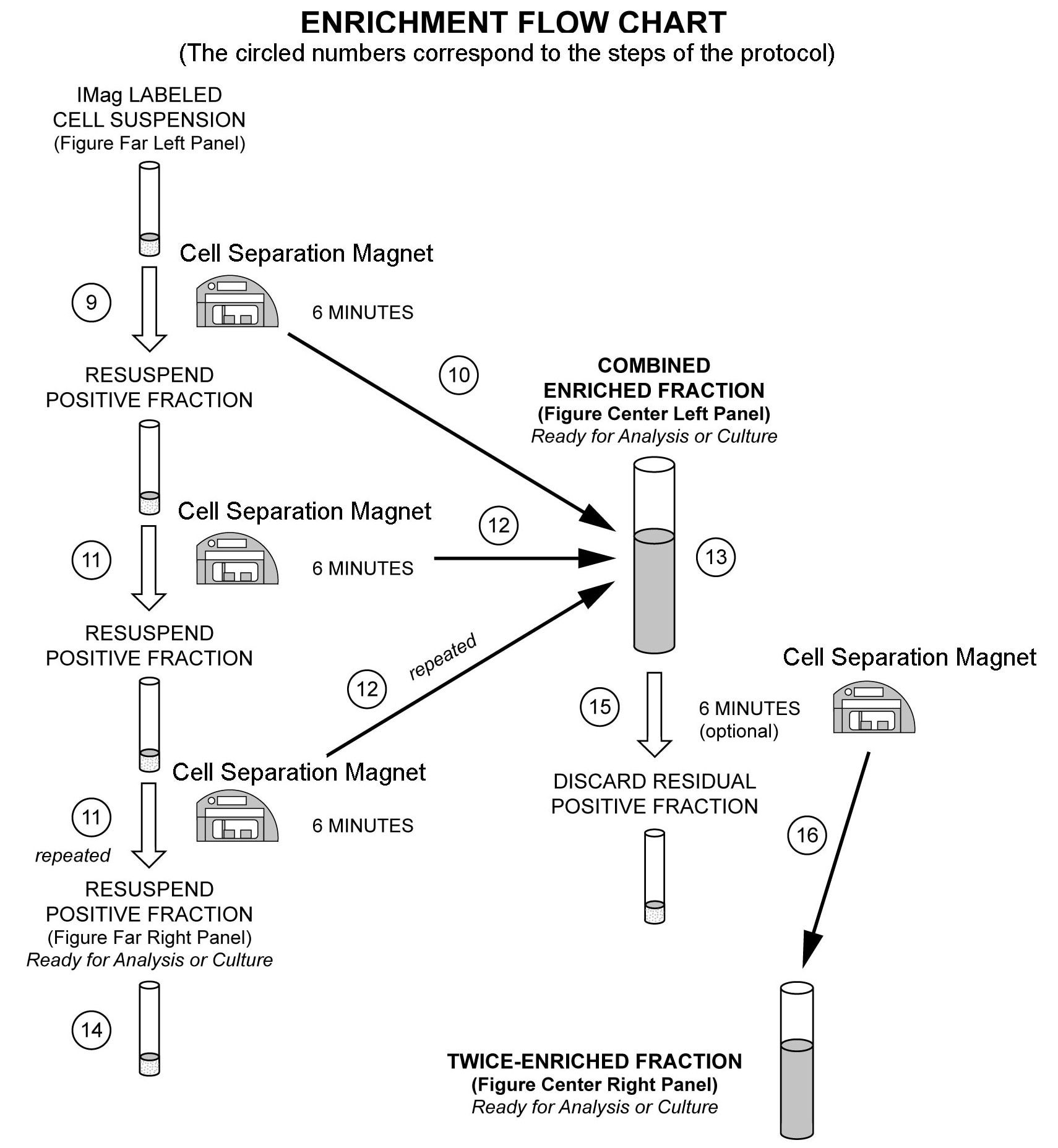
The detailed Magnetic Labeling and Enrichment Protocol follows. In summary, the Biotinylated Human NK Cell Enrichment Cocktail simultaneously stains erythrocytes, platelets, and most leukocytes except the NK cells. After washing away excess antibody, BD IMag™ Streptavidin Particles Plus - DM are added to the cell suspension and bind the cells bearing the biotinylated antibodies. The tube containing this labeled cell suspension is then placed within the magnetic field of the BD IMag™ Cell Separation Magnet (Cat. No. 552311). Negative selection is then performed to enrich for the unlabeled NK cells. Labeled cells migrate toward the magnet (positive fraction), leaving the unlabeled cells in suspension so they can be drawn off and retained (enriched fraction). The negative selection is repeated twice to increase the yield of the enriched fraction. If greater purity is required, negative selection may be performed on the enriched fraction. For clarification of the procedure, the magnetic separation steps are diagrammed in the Enrichment Flow Chart. The positive and enriched fractions can be evaluated in downstream applications such as flow cytometry and tissue culture. The antibodies in the Biotinylated Human NK Cell Enrichment Cocktail have been optimized and pre-diluted to provide maximum efficiency for enrichment of NK cells from PBMC.
MAGNETIC LABELING AND ENRICHMENT PROTOCOL
1. Prepare 1X BD IMag™ buffer: Dilute BD IMag™ Buffer (10X) (Cat. No. 552362) 1:10 with sterile distilled water or prepare Phosphate Buffered Saline (PBS) supplemented with 0.5% BSA, 2 mM EDTA, and 0.1% sodium azide.
1. Prepare PBMC from anti-coagulated human blood, preferably by density gradient centrifugation using Ficoll-Paque™.
2. Remove clumps of cells and/or debris by passing the suspension through a 70-µm nylon cell strainer. Count the cells, and res uspend them in 1X BD IMag™ buffer at a concentration of 10 x 10^6 cells/ml.
3. Add the Biotinylated Human NK Cell Enrichment Cocktail at 5 µl per 1 x 10^6 cells, and incubate at room temperature for 15 minutes.†
4. Wash the labeled cells with a 10X excess volume of 1X BD IMag™ buffer, centrifuge at 300 × g for 7 minutes, and carefully aspirate ALL the supernatant.
5. Vortex the BD IMag™ Streptavidin Particles Plus - DM thoroughly, and add 5 µl of particles for every 1 x 10^6 total cells.
6. MIX THOROUGHLY. Incubate at room temperature for 30 minutes.†
7. Bring the labeling volume up to a concentration of 20-80 x 10^6 cells/ml with 1X BD IMag buffer.
8. Transfer the labeled cells to a 12 x 75 mm round-bottom test tube, maximum volume added not to exceed 1.0 ml. Place this positive-fraction tube on the Cell Separation Magnet (horizontal position) for 6 to 8 minutes.
- For greater volume, transfer the cells to a 17 x 100 mm round-bottom test tube, maximum volume added not to exceed 3.0 ml. Place this positive-fraction tube on the Cell Separation Magnet (vertical position) for 8 minutes.
9. With the tube on the Cell Separation Magnet and using a sterile glass Pasteur pipette, carefully aspirate the supernatant (enriched fraction) and place in a new sterile tube.
10. Remove the positive-fraction tube from the Cell Separation Magnet, and add 1X BD IMag™ buffer to the same volume as in Step 8. Resuspend the positive fraction well by pipetting up and down 10 to 15 times, and place the tube back on the Cell Separation Magnet for 6 to 8 minutes.
- For 17 x 100 mm tube: Place on the Cell Separation Magnet for 8 minutes.
11. Using a new sterile Pasteur pipette, carefully aspirate the supernatant and combine with the enriched fraction from Step 10 above.
12. Repeat Steps 11 and 12. The combined enriched fraction contains NK cells with no bound antibodies or magnetic particles.
13. To increase the purity of the combined enriched fraction by another 5% or more (compare middle left and middle right panel in the figure), place the tube containing the combined enriched fraction on the Cell Separation Magnet for another 6 to 8 minutes.
- For 17 x 100 mm tube: Place on the Cell Separation Magnet for 8 minutes.
14. Carefully aspirate the supernatant and place in a new sterile tube. This is the twice-enriched fraction. The cells are ready to be processed for downstream applications.
15. The positive-fraction cells remaining in the original tube can be resuspended in an appropriate buffer or culture medium for downstream applications, including flow cytometry, if desired.
16. Samples of the total cell suspension and the positive and enriched fractions should be analyzed by flow cytometry to evaluate the efficiency of the cell-separation procedure.
* Hints for successful cell preparation:
- Draw the blood into a tube containing EDTA
- Remove the platelet rich plasma by centrifuging once at 220-240 × g.
- Wash 2-3 times in PBS after the density gradient separation.
- After the final wash, resuspend the cells at a relatively high concentration in 1X BD IMag™ buffer and proceed to Step 3.
† Avoid nonspecific labeling by working quickly and adhering to recommended incubation times.
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- BD IMag™ particles are prepared from carboxy-functionalized magnetic particles which are manufactured by Skold Technology and are licensed under US patent number 7,169,618.
- Ficoll-Paque is a trademark of Amersham Biosciences Limited.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
Companion Products

| Description | EntrezGene ID |
|---|---|
| Streptavidin Particles Plus - DM | N/A |
| Biotinylated Human NK Cell Enrichment Cocktail | N/A |
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Refer to manufacturer's instructions for use and related User Manuals and Technical Data Sheets before using this product as described.
Comparisons, where applicable, are made against older BD technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.