Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?



.png)

Analyses of Smad2 (pS465/pS467)/Smad3 (pS423/pS425) expression. Panel 1: Flow cytometric analysis of Ramos cells, human PBMC, and mouse spleen cells. Cells were cultured overnight in serum-free medium (Ramos, spleen) or medium containing 0.1% FBS (PBMC) and then either not treated (dashed line histogram) or treated with TGF-β (solid line histogram; Cat. No. 354039; 10 ng/mL, 30 min, 37°C). Cells were fixed in 1X BD Phosflow™ Lyse/Fix Buffer (Cat. No. 558049; 10 min, 37°C) and permeabilized in BD Phosflow™ Perm Buffer III (Cat. No. 558050; 30 min, on ice). Cells were stained with BD Phosflow™ PE-CF594 Mouse Anti-Smad2 (pS465/pS467)/Smad3 (pS423/pS425) antibody (Cat. No. 562697). PBMC were co-stained with PerCP-Cy™5.5 Mouse Anti-Human CD20 antibody (Cat. No. 558021). The fluorescence histograms were derived from events with the forward and side light-scatter characteristics of intact cells. Flow cytometry was performed using a BD™ LSR II Flow Cytometer System. Panel 2: Western blot analyses. Cells were serum-starved overnight and then either not treated (C) or treated with TGF-β (T), as in Panel 1. Lysates were blotted using Purified Mouse Anti-Smad2 (pS465/pS467)/Smad3 (pS423/pS425) antibody (Clone O72-670; 2 µg/mL). Phosphorylated Smad2 and Smad3 were identified as ~60 kDa and ~52 kDa bands, respectively. The lower molecular weight band detected in mouse spleen lysates is endogenous mouse Ig light chain. Panel 3: Immunohistochemical staining. An EDTA-pretreated, formalin-fixed, paraffin-embedded human tonsil section was stained with Ig Isotype Control (Cat. No. 550878) or Purified Mouse Anti-Smad2 (pS465/pS467)/Smad3 (pS423/pS425) antibody (Clone O72-670; 20X original magnification).

.png)

BD Phosflow™ PE-CF594 Mouse Anti-Smad2 (pS465/pS467)/Smad3 (pS423/pS425)

BD Phosflow™ PE-CF594 Mouse Anti-Smad2 (pS465/pS467)/Smad3 (pS423/pS425)
.png)
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
This fluorescent antibody is suitable for intracellular staining of human lymphoid cell lines, peripheral blood mononuclear cells, and mouse splenocytes using BD Cytofix™ Fixation Buffer or BD Phosflow™ Lyse/Fix Buffer with BD Phosflow™ Perm Buffer III (see table, below). Prior to stimulation, cells were serum starved overnight at a density of 2-10X10^6 cells/mL in flat-bottom 96- or 6-well tissue culture plates or in loosely capped, round-bottom tubes containing approximately 100 mL of cells in suspension.
Note:
1. Serum starvation for 2 hours following PBMC isolation was not sufficient to reduce basal phosphorylation of Smad2 and Smad3.
2. Do not mix or agitate untreated cells until just before the cells are ready to be fixed, since agitation of serum-starved mouse or human primary leukocytes prior to fixation increased Smad2/3 phosphorylation, even in the absence of exogenous TGF-β
Product Notices
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- Please observe the following precautions: Absorption of visible light can significantly alter the energy transfer occurring in any tandem fluorochrome conjugate; therefore, we recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to prevent exposure of conjugated reagents, including cells stained with those reagents, to room illumination.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Texas Red is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- CF™ is a trademark of Biotium, Inc.
- When excited by the yellow-green (561-nm) laser, the fluorescence may be brighter than when excited by the blue (488-nm) laser.
- This product is provided under an Agreement between BIOTIUM and BD Biosciences. The manufacture, use, sale, offer for sale, or import of this product is subject to one or more patents or pending applications owned or licensed by Biotium, Inc. This product, and only in the amount purchased by buyer, may be used solely for buyer’s own internal research, in a manner consistent with the accompanying product literature. No other right to use, sell or otherwise transfer (a) this product, or (b) its components is hereby granted expressly, by implication or by estoppel. This product is for research use only. Diagnostic uses require a separate license from Biotium, Inc. For information on purchasing a license to this product including for purposes other than research, contact Biotium, Inc., 3159 Corporate Place, Hayward, CA 94545, Tel: (510) 265-1027. Fax: (510) 265-1352. Email: btinfo@biotium.com.
- Because of the broad absorption spectrum of the tandem fluorochrome, extra care must be taken when using multi-laser cytometers, which may directly excite both PE and CF™594.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Species cross-reactivity detected in product development may not have been confirmed on every format and/or application.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products

The O72-670 monoclonal antibody specifically binds to the Smad2 protein phosphorylated at the Ser465/467 sites and the Smad3 protein phosphorylated at the Ser423/425 sites. Smad2 and Smad3 are members of the Smad superfamily with observed molecular weights of 60 kDa and 52 kDa, respectively. The Smad family consists of three subfamilies: receptor regulated Smads or R-Smads, including Smads1, 2, 3, 5, and 8; common partner Smad, or Co-Smad, including Smad4; and inhibitory Smads, or I-Smad, including Smads 6 and 7. Activation of TGF-beta superfamily serine/threonine kinase receptors, such as TGF-beta, activin and BMP receptors, by their bound ligands leads to the phosphorylation of R-Smads at several sites. It has been shown that the ligand-bound TGF-beta type I receptor directly phosphorylates Ser465 and Ser467 of Smad2 and Ser423 and Ser425 of Smad3. Phosphorylated R-Smads form complexes with Co-Smad and translocate into the nucleus to regulate transcription affecting a wide range of critical cellular processes including cell-fate determination, proliferation, morphogenesis, differentiation and apoptosis. The inhibitory Smads inhibit this pathway through two potential mechanisms: either by preventing R-Smads from binding to their corresponding receptors and/or by competing with Smad4, the Co-Smad, from binding to R-Smads. High level expression of phosphorylated Smad2 has been associated with poor prognosis in late stage gastric carcinoma. Roles for Smad2 have been described in thymopoiesis and the TGF-β-mediated induction of regulatory T cells and Th17 cells. The specificity of the O72-670 mAb was confirmed by Western blot and immunohistochemistry using unconjugated antibody.
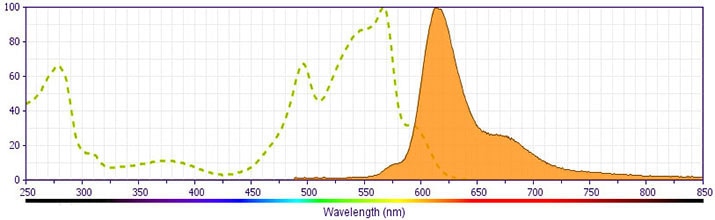
This antibody is conjugated to BD Horizon™ PE-CF594, which has been developed exclusively by BD Biosciences as a better alternative to PE-Texas Red®. PE-CF594 excites and emits at similar wavelengths to PE-Texas Red® yet exhibits improved brightness and spectral characteristics. Due to PE having maximal absorption peaks at 496 nm and 564 nm, PE-CF594 can be excited by the blue (488-nm), green (532-nm) and yellow-green (561-nm) lasers and can be detected with the same filter set as PE-Texas Red® (eg 610/20-nm filter).
Development References (7)
-
Abdollah S, Macías-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana JL. TbetaRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. J Biol Chem. 1997; 272(44):27678-27685. (Biology). View Reference
-
Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997; 390(6659):465-471. (Biology). View Reference
-
Martinez GJ, Zhang Z, Reynolds JM, et al. Smad2 positively regulates the generation of Th17 cells. J Biol Chem. 2010; 285(38):29039-29043. (Biology). View Reference
-
Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001; 114(24):4359-69. (Biology). View Reference
-
Rosendahl A, Speletas M, Leandersson K, Ivars F, Sideras P. Transforming growth factor-beta- and Activin-Smad signaling pathways are activated at distinct maturation stages of the thymopoeisis. Int Immunol. 2003; 15(12):1401-1414. (Biology). View Reference
-
Shinto O, Yashiro M, Toyokawa T, et al. Phosphorylated Smad2 in advanced stage gastric carcinoma. BMC Cancer. 2010; 10:652. (Biology). View Reference
-
Souchelnytskyi S, Tamaki K, Engström U, Wernstedt C, ten Dijke P, Heldin CH. Phosphorylation of Ser465 and Ser467 in the C terminus of Smad2 mediates interaction with Smad4 and is required for transforming growth factor-beta signaling. J Biol Chem. 1997; 272(44):28107-28115. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Refer to manufacturer's instructions for use and related User Manuals and Technical Data Sheets before using this product as described.
Comparisons, where applicable, are made against older BD technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.