Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD™ Stem Cell Enumeration Kit
(IVD)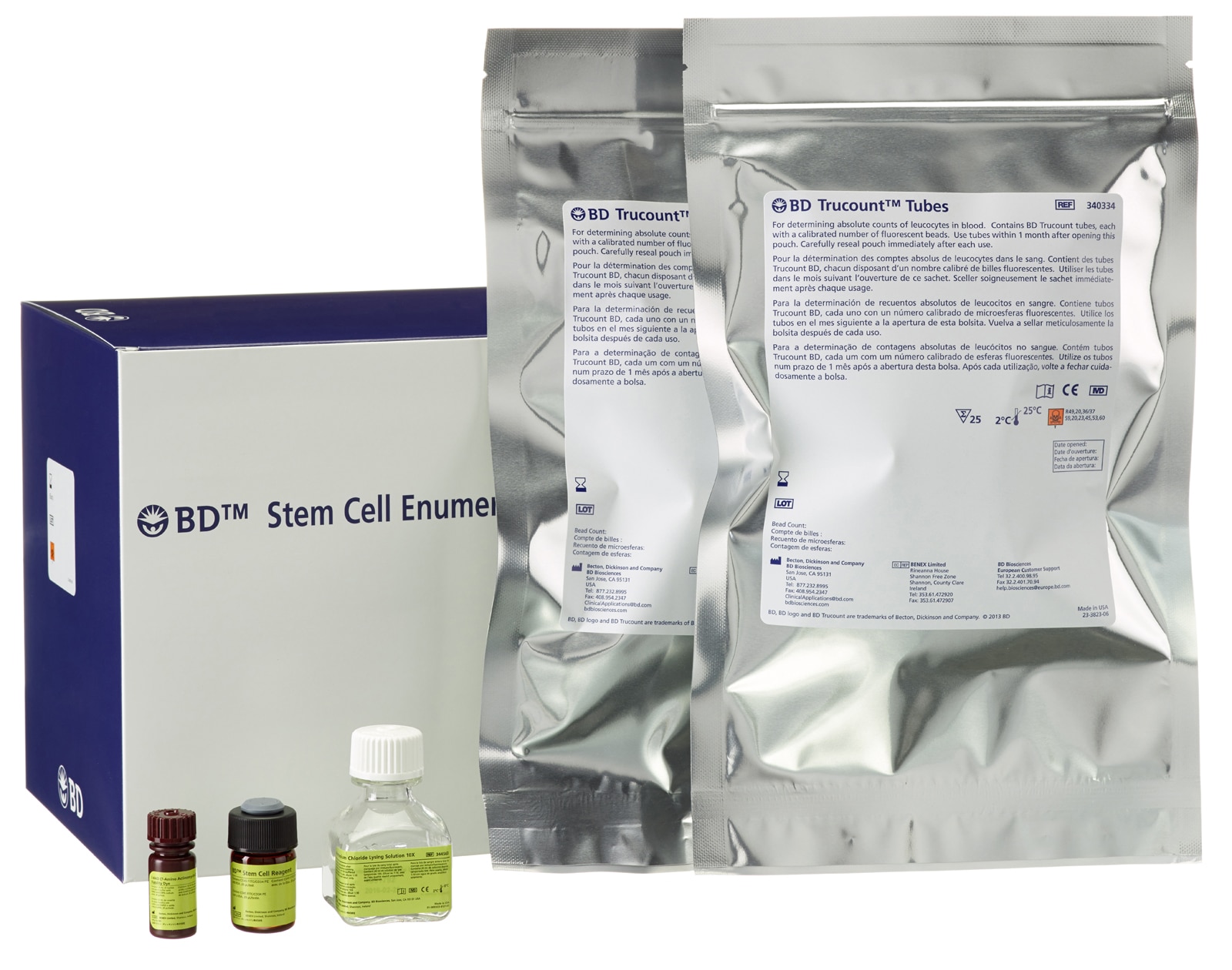

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
The BD™ Stem Cell Enumeration (SCE) kit provides simultaneous enumeration of viable dual-positive CD45+/CD34+ hematopoietic stem cell populations in CD34+ absolute counts (cells/µL) as well as the percentage of the total viable leucocyte count that is CD34+ (%CD34). The following specimens can be analyzed with this kit: normal and mobilized peripheral blood, fresh and thawed leucopheresis products, fresh and thawed bone marrow, and fresh and thawed cord blood. The kit is intended for in vitro diagnostic (IVD) use on a BD FACSLyric™ Flow Cytometer using BD FACSuite™ Clinical Application or a BD FACSCanto™ II Flow Cytometer using BD FACSCanto™ Clinical Software or a BD FACSCalibur™ Flow Cytometer using BD CellQuest™ or BD CellQuest™ Pro Software. Contents: BD Stem Cell reagent (CD45 FITC/CD34 PE), 7-aminoactinomycin-D (7-AAD) reagent, 10X ammonium chloride lysing solution, 50 BD Trucount tubes.
Preparation And Storage
• Store the BD Stem Cell (CD45/CD34) reagent at 2°C to 8°C. Do not use after the expiration date shown on the label. Do not freeze the reagent or expose it to direct light during storage or incubation with cells. Keep the reagent vial dry.
• Store BD Trucount tubes in their original foil pouch at 2°C to 25°C. To avoid potential condensation, open the pouch only after it has reached room temperature and carefully reseal the pouch immediately after removing a tube. Examine the desiccant each time you open the pouch. If the desiccant has turned from blue to lavender, discard the remaining tubes. An unopened pouch is stable until the expiration date shown on the packaging. Do not open and use tubes after this expiration date. Use tubes within 1 hour after removal from the foil pouch. Use remaining tubes within 1 month after opening the pouch.
• Store 7-AAD at 2°C to 8°C. Protect from light.
• Store 10X ammonium chloride at 2°C to 8°C.
| Description | Quantity/Size | Part Number | EntrezGene ID |
|---|---|---|---|
| BD Stem Cell reagent (CD45 FITC/CD34 PE) | 50 Tests (1 ea) | 91-0674 | N/A |
| 7-aminoactinomycin-D (7-AAD) reagent | 50 Tests (1 ea) | 91-0675 | N/A |
| 10X ammonium chloride lysing solution | 50 Tests (1 ea) | 91-0693 | N/A |
| BD Trucount tubes (25 tubes) | N/A | 91-0786 | N/A |
Development References (18)
-
Antonenas V, Garvin F, Webb M, Sartor M, Bradstock KF, Gottlieb D. Fresh PBSC harvests, but not BM, show temperature-related loss of CD34 viability during storage and transport. Cytometry. 2006; 8:158-165. (Biology).
-
Brocklebank AM, Sparrow RL. Enumeration of CD34+ cells in cord blood: a variation on a single-platform flow cytometric method based on the ISHAGE gating strategy.. Cytometry. 2001; 46(4):254-61. (Biology). View Reference
-
Centers for Disease Control. Update: universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in healthcare settings. MMWR. 1988; 37:377-388. (Biology).
-
Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF, Shaper JH. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. Civin CI, Strauss LC; 133(1):157-165. (Biology). View Reference
-
Civin CI, Trischmann TM, Fackler MJ, et al. Knapp W, Dörken B, Gilks WR, et al, ed. Leucocyte Typing IV: White Cell Differentiation Antigens. New York, NY: Oxford University Press; 1989:818-825.
-
Clinical Applications of Flow Cytometry: Quality Assurance and Immunophenotyping of Lymphocytes: Approved Guideline. H42-A2. 2007. (Biology).
-
Clinical and Laboratory Standards Institute. 2005. (Biology).
-
Cobbold SP, Hale G, Waldmann H. Non-lineage, LFA-1 family, and leucocyte common antigens: new and previously defined clusters. In: McMichael AJ. A.J. McMichael .. et al., ed. Leucocyte typing III : white cell differentiation antigens. Oxford New York: Oxford University Press; 1987:788-803.
-
Greaves MF, Titley I, Colman SM, et al. CD34 cluster workshop report. In: Schlossman SF. Stuart F. Schlossman .. et al., ed. Leucocyte typing V : white cell differentiation antigens : proceedings of the fifth international workshop and conference held in Boston, USA, 3-7 November, 1993. Oxford: Oxford University Press; 1995:840-846.
-
Jackson AL, Warner NL. Rose NR, Friedman H, Fahey JL, ed. Manual of Clincial Laboratory Immunology, Third Edition. Washington DC: American Society for Microbiology; 1986:226-235.
-
Keeney M, Brown W, Gratama J, Papa S, Lanza F, Sutherland DR. Single platform enumeration of viable CD34pos cells. J Biol Regul Homeost Agents. 2003; 17:247-253. (Biology).
-
Langenmayer I, Weaver C, Buckner CD, et al. Engraftment of patients with lymphoid malignancies transplanted with autologous bone marrow, peripheral blood stem cells or both. Bone Marrow Transplant. 1995; 15:241-246. (Biology).
-
Loken MR, Shah VO, Dattilio KL, Civin CI. Flow cytometric analysis of human bone marrow. II. Normal B lymphocyte development. Blood. 1987; 70(5):1316-1324. (Biology). View Reference
-
Schwinzer R. Cluster Report: CD45/CD45R. In: Knapp W. W. Knapp .. et al., ed. Leucocyte typing IV : white cell differentiation antigens. Oxford New York: Oxford University Press; 1989:628-634.
-
Shpall EJ, Jones RB, Bearman SI, et al. Transplantation of enriched CD34-positive autologous marrow into breast cancer patients following high-dose chemotherapy: influence of CD34-positive peripheral-blood progenitors and growth factors on engraftment. J Clin Oncol. 1994; 12:28-36. (Biology).
-
Siena S, Bregni M, Brando B, et al. Flow cytometry for clinical estimation of circulating hematopoietic progenitors for autologous transplantation in cancer patients.. Blood. 1991; 77(2):400-9. (Biology). View Reference
-
Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. J Hematotherapy. 1996; 5:213-226. (Biology).
-
Zander AR, Lyding J, Bielack S. Transplantation with blood stem cells. Blood Cells. 1991; 17:301-309. (Biology).
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For In Vitro Diagnostics Use.
Refer to manufacturer's instructions for use and related User Manuals and Technical Data Sheets before using this product as described.
Comparisons, where applicable, are made against older BD technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.