-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- Advanced Training
-
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?





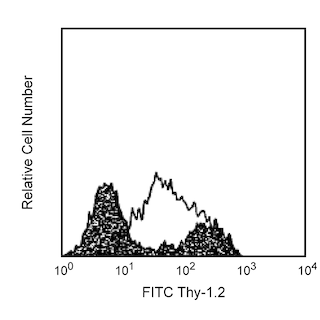
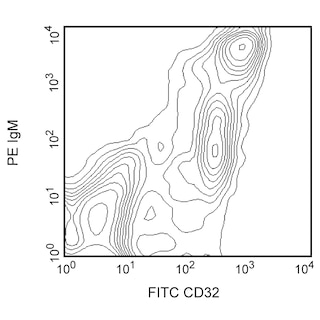
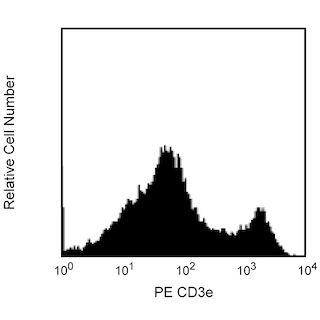
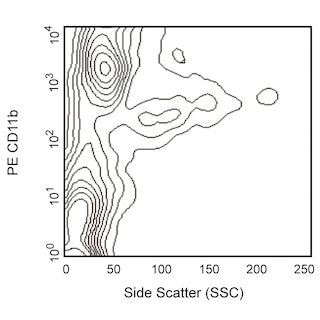
Figure 1. Depletion of T-, B-, NK-, and myeloid-lineage cells from mouse bone marrow. BALB/c bone-marrow cells (BM) were labeled with PE Rat Anti-Mouse CD11b mAb (Cat. No. 553311), CD45R/B220 mAb (Cat. No. 553089), and Ly-6G and Ly-6C mAb (Cat. No. 553128) as well as PE Hamster Anti-Mouse CD3e mAb (Cat. No. 553063), followed by BD IMag™ Anti- R-Phycoerythrin (PE) Magnetic Particles - DM (Cat. No. 557899), then separated using the BD IMag™ Cell Separation Magnet (Cat. No. 552311) according to the accompanying depletion protocol. Please refer to the Depletion Flow Chart to identify the separated cell populations represented in this figure. The percentage of positive cells is indicated in each panel; placement of each marker is based upon staining with the appropriate isotype control (data not shown). The negative or unstained cells seen in the left and middle panels are primarily erythroid cells. Flow cytometry was performed on a BD FACSCalibur™ flow cytometry instrument. Figure 2. Positive selection of human γδ TCR-bearing T lymphocytes. Peripheral blood mononuclear cells (PBMC) were stained with PE Mouse Anti-Human γδ TCR mAb (Cat. No. 555717), labeled with BD IMag™ Anti- R-Phycoerythrin (PE) Magnetic Particles - DM, then separated using the Cell Separation Magnet. The negative (γδ TCR-) and positive (γδ TCR+) fractions were collected as described in the positive selection protocol. Please refer to the accompanying Positive Selection Flow Chart to identify the separated cell populations represented in this figure. For flow cytometric analysis, cells were stained with APC mouse anti-human CD3 mAb (Cat. No. 555335).The percentage of CD3+ γδ TCR+ T lymphocytes in each sample is given. Flow cytometry was performed on a BD FACSCalibur™ flow cytometry instrument.

Table 1. Various reported optimal concentrations of BD IMag™ Anti- R-Phycoerythrin (PE) Magnetic Particles - DM for positive selection with some PE-conjugated monoclonal antibodies to human and mouse leukocyte antigens.


BD IMag™ Anti- R-Phycoerythrin (PE) Magnetic Particles - DM

BD IMag™ Anti- R-Phycoerythrin (PE) Magnetic Particles - DM

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
MAGNETIC LABELING AND SEPARATION PROTOCOL
1. Prepare the following buffers and place on ice:
a. Cell-staining buffer: Phosphate Buffered Saline, 3% heat inactivated fetal calf serum, 0.1% sodium azide.
b. 1X BD IMag™ buffer: Dilute BD IMag™ Buffer (10X) (Cat. no. 552362) 1:10 with sterile distilled water or alternatively, prepare Phosphate Buffered Saline, supplemented with 0.5% BSA, 2 mM EDTA, and 0.1% sodium azide.*
2. Prepare a single-cell suspension from the lymphoid tissue of interest or prepare PBMC from anti-coagulated blood, preferably by density gradient centrifugation using the appropriate density Ficoll-Hypaque™ solution. Remove clumps of cells and/or debris by passing the suspended cells through a 70-µm nylon cell strainer.
3. Count the cells, and resuspend them in cell-staining buffer at a concentration of 2 x 10e7 cells/ml.
4. Optional: If appropriate, add BD Mouse Fc Block™ Purified Anti-Mouse CD16/CD32 (Cat. No. 553141) or BD Rat Fc Block™ Purified Anti-Rat CD32 (Cat. No. 550270) at 0.25 µg/10e6 cells, and incubate on ice for 15 minutes.
5. Add the PE-conjugated antibody (or antibody cocktail) at the appropriate concentration, and incubate on ice for 15 minutes. †
6. Wash the labeled cells with an excess volume of 1X BD IMag™ buffer, and carefully aspirate ALL the supernatant. For depletions, proceed with Step 7. For positive selections, proceed with Step 18.
Depletions:
7. Vortex the Anti- R-Phycoerythrin (PE) Magnetic Particles - DM thoroughly, and add 50 µl of particles per 1x10e7 total cells.
8. MIX THOROUGHLY. Incubate mouse/ rat leukocytes for 30 minutes at 6°C-12°C and human PBMC at room temperature for 30 minutes. †
9. Bring the labeling volume up to a concentration of 2-8 x 10e7 cells/ml with 1X BD IMag™ buffer or culture medium.*
10. Transfer the labeled cells to a 12 x 75 mm round-bottom test tube, maximum volume added not to exceed 1.0 ml. Place this positive-fraction tube on the Cell Separation Magnet (horizontal position) for 6-8 minutes.
- For greater volume, transfer the cells to a 17 x 100 mm round-bottom test tube maximum volume added not to exceed 3.0 ml. Place this positive-fraction tube on the Cell Separation Magnet (vertical position) for 8 minutes.
11. With the tube on the Cell Separation Magnet, use a glass Pasteur pipette to aspirate the supernatant (depleted fraction) into a new tube.
12. Remove the positive fraction tube from the Cell Separation Magnet, and add 1X BD IMag™ buffer (or medium) to the same volume as in Step 9. Resuspend the positive fraction well by pipetting up and down 10-15 times and place back on the Cell Separation Magnet for 6-8 minutes.
- 17 x 100 mm tube: Place on the Cell Separation Magnet for 8 minutes.
13. Using a new Pasteur pipette, carefully aspirate the supernatant and combine with the depleted fraction from Step 11 above.
14. Repeat Steps 12 and 13. The combined depleted fraction contains cells with no bound antibodies or magnetic particles. These cells are ready for downstream applications, or they can be further enriched by proceeding to Step 16.
15. The positive-fraction cells remaining in the original tube can be resuspended in an appropriate buffer or culture medium for downstream applications, including flow cytometry.
16. To increase the purity of the combined depleted fraction, place the tube on the Cell Separation Magnet for another 6-8 minutes.
- 17 x 100 mm tube: Place on the Cell Separation Magnet for 8 minutes.
17. Carefully aspirate the supernatant (final depleted fraction) into a new tube. The cells are ready to be processed for downstream applications.
Positive Selections:
18. Vortex the Anti- R-Phycoerythrin (PE) Magnetic Particles - DM thoroughly, and add 10-50 µl of particles per1 x 10e7 total cells.
Note: The amount of particles to add may vary depending on how many cells one is targeting and the cell-surface density of the antigen. Please refer to Table 1 for some common examples.
19. MIX THOROUGHLY. Incubate mouse/ rat leukocytes for 30 minutes at 6°C - 12°C and human PBMC at room temperature for 30 minutes. †
20. Bring the labeling volume up to a concentration of 2-8 x 10e7 cells/ml with 1X BD IMag™ buffer.
21. Immediately place the tube onto the Cell Separation Magnet and incubate for 6-8 minutes.
22. With the tube on the Cell Separation Magnet, carefully aspirate the supernatant. This supernatant is the negative fraction.
23. Remove the tube from the Cell Separation Magnet, and add 1X BD IMag™ buffer to the same volume as in Step 20. Gently resuspend the cells by pipetting up and down, and return the tube to the Cell Separation Magnet for another 2-4 minutes.
24. With the tube on the Cell Separation Magnet, carefully remove the supernatant.
25. Repeat Steps 23 and 24.
26. After the final wash step, remove the tube from the Cell Separation Magnet. Resuspend the Positive Fraction in an appropriate buffer or culture medium, and proceed with desired downstream application(s), including flow cytometry.
NOTES: * For depletion of mouse leukocytes, tissue culture medium usually results in a slight increase in viability and recovery, when compared to IMag buffer, without reducing cell purity. Because applications can vary, researchers are encouraged to run a trial comparison of culture media and IMag buffer to demonstrate that there are no adverse effects.
† Avoid non-specific labeling by working quickly and adhering to recommended incubation times.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- BD IMag™ particles are prepared from carboxy-functionalized magnetic particles which are manufactured by Skold Technology and are licensed under US patent number 7,169,618.
- Ficoll-Paque is a trademark of Amersham Biosciences Limited.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products

BD IMag™ Anti- R-Phycoerythrin (PE) Magnetic Particles - DM are magnetic nanoparticles that have monoclonal antibody conjugated to their surfaces. The E31-1459 antibody clone reacts with PE, a commonly used fluorochrome for flow cytometry. The binding of the E31-1459 antibody to PE has been reported not to quench the fluorescence of the PE molecule. These magnetic particles are optimized for the positive selection or depletion of leukocyte subpopulations using the BD IMag™ Cell Separation Magnet.
Leukocytes are labelled with BD IMag™ Anti- R-Phycoerythrin (PE) Magnetic Particles - DM according to the Magnetic Labeling and Separation Protocol. In brief, cells are labeled with a PE-conjugated antibody that recognizes the subpopulation of interest. After washing away excess antibody, BD IMag™ Anti- R-Phycoerythrin (PE) Magnetic Particles - DM are added to the cell suspension and bind the PE-conjugated antibody on the cells. This labeled cell suspension is then placed within the magnetic field of the Cell Separation Magnet. Positive selection or depletion is then performed. Labeled cells migrate toward the magnet (positive fraction), leaving the unlabeled cells in suspension so they can be drawn off (depleted or negative fraction). The tube is then removed from the magnetic field for resuspension of the positive fraction. The selections are repeated twice to increase the purity of the positive fraction and the yield of the depleted fraction. The magnetic separation steps are diagrammed in the Depletion and Positive Selection Flow Charts. After the positive fraction has been washed, the small size of the magnetic particles allows the positive fraction to be further evaluated in downstream applications such as flow cytometry.
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.