-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- Advanced Training
-
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?




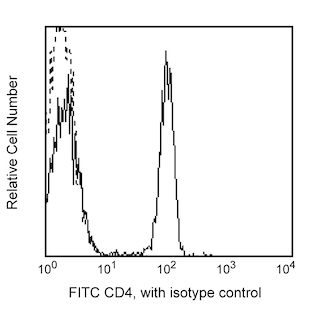
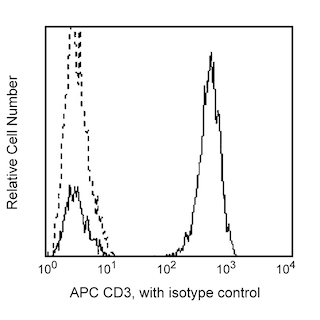
Positive selection of human CD4+ T lymphocytes from PBMC. Leukocytes were labeled with BD IMag™ anti-human CD4 Particles - DM (Cat. No. 557767) as described in the protocol. After labeling, the cells were separated using the BD IMag™ Cell Separation Magnet (Cat. No. 552311) and the negative (CD4-) and positive (CD4+) fractions were collected. Please refer to the Separation Flow Chart to identify the separated cell populations represented in this figure. For flow cytometric analysis, fresh PBMC (left panel), the negative fraction (middle fraction), and the positive fraction (right panel) were stained with FITC Mouse Anti-Human CD4 (Cat. No. 555346) and APC Mouse Anti-Human CD3 (Cat. No. 555335). The percent CD4+/CD3+ cells in each sample is given.



BD IMag™ Anti-Human CD4 Particles - DM

BD IMag™ Anti-Human CD4 Particles - DM

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Peripheral Blood Mononuclear Cells (PBMC) are labeled with BD IMag™ anti-human CD4 Particles - DM according to the following Protocol. This labeled cell suspension is then placed within the magnetic field of the BD IMag™ Cell Separation Magnet (Cat. No. 552311). Labeled cells migrate toward the magnetic (positive fraction), leaving the unlabeled cells in suspension so they can be drawn off (negative fraction). The tube is then removed from the magnetic field for resuspension of the positive fraction. The separation is repeated twice to increase the purity of the positive fraction. The magnetic separation steps are diagrammed in the Separation Flow Chart. After the positive fraction is washed, the small size of the magnetic particles allows the positive fraction to be further evaluated in downstream applications such as flow cytometry.
MAGNETIC LABELING PROTOCOL
1. Prepare PBMC from anti-coagulated human (or rhesus macaque) blood, preferably by density gradient centrifugation using Ficoll-Paque™.*
2. Dilute BD IMag™ Buffer (10X) (Cat. No. 552362) 1:10 with sterile distilled water or prepare 1X BD IMag™ buffer by supplementing Phosphate Buffered Saline with 0.5% BSA, 2 mM EDTA, and 0.09% sodium azide). Store at 4°C.
3. Count cells, wash them with an excess volume of 1X BD IMag™ buffer, and carefully aspirate all the supernatant.
4. Vortex the BD IMag™ anti-human CD4 Particles - DM thoroughly, and add 50 µl of particles for every 10^7 total cells.†
5. MIX THOROUGHLY. Incubate at room temperature for 30 minutes. ‡
6. Bring the BD IMag™-particle labeling volume up to 1 - 8 x 10^7 cells/ml with 1X BD IMag buffer, and immediately place the tube on the Cell Separation Magnet. Incubate for 8 - 10 minutes.
7. With the tube on the Cell Separation Magnet, carefully aspirate off the supernatant. This supernatant contains the negative fraction.
8. Remove the tube from the Cell Separation Magnet, and add 1 ml of 1X BD IMag™ buffer to the same volume as in Step 6. Gently resuspend cells by pipetting up and down, and return the tube to the Cell Separation Magnet for another 2 - 4 minutes.
9. With the tube on the Cell Separation Magnet, carefully aspirate off the supernatant and discard.
10. Repeat Steps 8 and 9.
11. After the final wash step, resuspend the positive fraction in an appropriate buffer or media, and proceed with desired downstream application(s).
NOTES:
* Hints for successful cell preparation:
-Draw the blood into a tube containing EDTA.
-Remove the platelet rich plasma by centrifuging once at 220-240 × g.
-Wash 2-3 times in PBS after the density gradient separation.
-Remove clumps of cells and/or debris by passing the suspension through a 70-µm nylon cell strainer.
† The BD IMag™ particles may need to be titrated to optimize the separation of rhesus macaque leukocytes.
‡ Avoid nonspecific labeling by working quickly and adhering to the recommended incubation times.
Product Notices
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- BD IMag™ particles are prepared from carboxy-functionalized magnetic particles which are manufactured by Skold Technology and are licensed under US patent number 7,169,618.
- Ficoll-Paque is a trademark of Amersham Biosciences Limited.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products

BD IMag™ anti-human CD4 Particles - DM are magnetic nanoparticles that have monoclonal antibody conjugated to their surfaces. These particles are optimized for the positive selection or depletion of CD4-bearing T lymphocytes using the BD IMag™ Cell Separation Magnet. The L200 antibody reacts with CD4 on human, rhesus and cynomolgus macaque, and baboon peripheral blood leukocytes; we have confirmed that the BD IMag™ particles can effectively separate the CD4-bearing cells of rhesus macaque blood. The distribution of CD4 on peripheral leukocytes is similar for both human and monkey. It is on the MHC class II-restricted T helper cells, with the majority of CD4-positive lymphocytes being CD8-negative. It is also found on most thymocytes and at low density on monocytes; it is not found on B or NK cells.
Development References (11)
-
Bleavins MR, Brott DA, Alvey JD, de la Iglesia FA. Flow cytometric characterization of lymphocyte subpopulations in the cynomolgus monkey (Macaca fascicularis). Vet Immunol Immunopathol. 1993; 37(1):1-13. (Biology). View Reference
-
Giorgi JV, Hultin LE, Desrosiers RC. The immunopathogenesis of retroviral diseases: no immunophenotypic alterations in T, B, and NK cell subsets in SIVmac239-challenged rhesus macaques protected by SIV delta nef vaccination. J Med Primatol. 1996; 25(3):186-191. (Biology). View Reference
-
Indzhiia LV, Yakovleva LA, Overbaugh J, et al. Baboon T cell lymphomas expressing the B cell-associated surface proteins CD40 and Bgp95. J Clin Invest. 1992; 12(3):225-236. (Biology). View Reference
-
Jacobsen CN, Aasted B, Broe MK, Petersen JL. Reactivities of 20 anti-human monoclonal antibodies with leucocytes from ten different animal species. Vet Immunol Immunopathol. 1993; 39(4):461-466. (Biology). View Reference
-
Knapp W. W. Knapp .. et al., ed. Leucocyte typing IV : white cell differentiation antigens. Oxford New York: Oxford University Press; 1989:1-1182.
-
Powell JD, McClure HM, Anderson D, Fultz PN, Sell KW, Ahmed-Ansari A. Phenotypic and functional differences in NK and LAK cells in the peripheral blood of sooty mangabeys and rhesus macaques. Cell Immunol. 1989; 124(1):107-118. (Biology). View Reference
-
Savary CA, Lotzova E, Jackson HJ, Jardine JH, Ang KK. Analysis of interleukin-2-activated killer cells of rhesus monkeys: striking resemblance to the human system. J Leukoc Biol. 1993; 54(4):307-313. (Biology). View Reference
-
Schlossman SF. Stuart F. Schlossman .. et al., ed. Leucocyte typing V : white cell differentiation antigens : proceedings of the fifth international workshop and conference held in Boston, USA, 3-7 November, 1993. Oxford: Oxford University Press; 1995.
-
Tryphonas H, Lacroix F, Hayward S, Izaguirre C, Parenteau M, Fournier J. Cell surface marker evaluation of infant Macaca monkey leukocytes in peripheral whole blood using simultaneous dual-color immunophenotypic analysis. J Med Primatol. 1996; 25(2):89-105. (Biology). View Reference
-
Verdier F, Aujoulat M, Condevaux F, Descotes J. Determination of lymphocyte subsets and cytokine levels in cynomolgus monkeys. Toxicology. 1995; 105(1):81-90. (Biology). View Reference
-
Wilson AD, Shooshtari M, Finerty S, Watkins P, Morgan AJ. Selection of monoclonal antibodies for the identification of lymphocyte surface antigens in the New World primate Saguinus oedipus oedipus (cotton top tamarin). J Immunol Methods. 1995; 178(2):195-200. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.