-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- Advanced Training
-
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Stemflow™ Human Neural Cell Sorting Kit
(RUO)


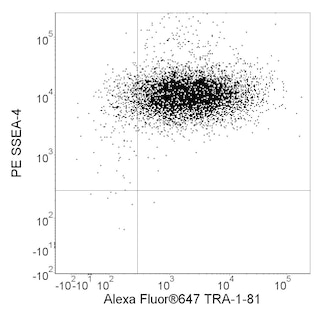
Figure 1 Cell sorting of NSC from neural induction cultures: H9 hESC were differentiated into neural ectoderm using SFEB and dual SMAD inhibition. Please see Recommended Assay Procedure for more details. On day 20 of differentiation cells were processed as described above and sorted using the following gating strategy. Neural Stem Cell Sort (Gates based upon isotype controls): 1. Identify cells in FSC v SSC plot. (P1) a. Doublet discrimination should be applied after this step (P2) 2. Create a child gate from P2 that includes the CD184+ cells (P3). 3. Create a child gate from P3 that includes CD44- and CD271- cells (P4) 4. Create a child gate from P4 that in cludes the CD24+CD15+/- (NSC) cells Figure 2 Cell sorting of neurons and glia from differentiating NSC cultures: Previously sorted and expanded NSC derived from H9 hESC were differentiated for ~ 2.5 weeks in DMEM:F12+Glutamax, 1X B27, 1X N2 (Invitrogen), 1X P/S (Lonza), 20 ng/ml BDNF, 20ng/ml GDNF (both from Peprotech) and 0.5 mM dibutyryl cyclic AMP (Sigma). Cells were processed as described above and sorted using the following gating strategy. Neuron and Glia Sort (Gates based upon isotype controls): 1. Identify cells in FSC v SSC plot. (P1) a. Doublet discrimination should be applied after this step. (P2) b. Make sure to include the glia as they appear as a distinct scatter population in more mature cultures. 2. Create a child gates from P2 that includes CD44-CD184- cells (P3) or CD44+CD184+ cells (glia) 3. Create a child gate from P3 that includes the CD24+CD15- cells (Neurons)

BD Stemflow™ Human Neural Cell Sorting Kit
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
The BD Stemflow™ Neural Cell Isolation Kit was designed to allow the isolation of neural stem cells (NSCs) derived from human pluripotent stem cells or the isolation of neurons and glial cells from differentiated NSCs. Following a variety of established neural induction protocols, NSCs are sorted from the heterogeneous cell culture utilizing the included antibody conjugates to five cell surface markers. This method greatly reduces the amount of variability between NSC isolations as opposed to traditional methods like manual isolation. In addition, four of the five included conjugates can be used to isolate neurons from glia from differentiated NSCs.
Kit Components
Component Description Size Vol. Per Test Storage Buffer
51-9007757 PE Mouse Anti-Human CD24 20 Test 5 µl Aqueous buffered solution containing
BSA and <0.09% sodium azide
51-9007758 PerCP-Cy™5.5 Mouse Anti-Human CD271 20 Test 5 µl Aqueous buffered solution containing
BSA and <0.09% sodium azide
51-9007759 PerCP-Cy™5.5 Mouse Anti-Human CD44 20 Test 5 µl Aqueous buffered solution containing
BSA and <0.09% sodium azide
51-9007760 PE-Cy™7 Mouse Anti-Human CD15 20 Test 5 µl Aqueous buffered solution containing
BSA and <0.09% sodium azide
51-9007761 APC Mouse Anti-Human CD184 20 Test 5 µl Aqueous buffered solution containing
BSA and <0.09% sodium azide
51-9007762 PE Mouse IgG1, κ Isotype Control 10 Test 5 µl Aqueous buffered solution containing
BSA and <0.09% sodium azide
51-9007763 PerCP-Cy™5.5 Mouse IgG1, κ 10 Test 5 µl Aqueous buffered solution containing
Isotype Control for 51-007758 BSA and <0.09% sodium azide
51-9007764 PerCP-Cy™5.5 Mouse IgG1, κ 10 Test 5 µl Aqueous buffered solution containing
Isotype Control for 51-9007759 BSA and <0.09% sodium azide
51-9007765 PE-Cy™7 Mouse IgM, κ Isotype Control 10 Test 5 µl Aqueous buffered solution containing
BSA and <0.09% sodium azide
51-9007766 APC Mouse IgG1, κ Isotype Control 10 Test 5 µl Aqueous buffered solution containing
BSA and <0.09% sodium azide
51-9007767 Negative Control CompBead Plus 1.5 ml 1 drop Aqueous buffered solution containing
BSA and <0.09% sodium azide
51-9007768 Anti-Mouse Ig, κ CompBead Plus 1.5 ml 1 drop Aqueous buffered solution containing
BSA and <0.09% sodium azide
Specificity Clone Molecule NSC Phenotype Neuron Phenotype Glial Phenotype
CD15 HI98 X-hapten, SSEA-1 -/+ Low NA
CD24 ML5 Heat Stable Antigen + + NA
CD44 G44-26 H-CAM - - +
CD184 12G5 CXCR4, Fusin + - +
CD271 C40-1457 NGF-Receptor - NA NA
Preparation And Storage
Recommended Assay Procedures
We have had consistent results with the H9 (WiCell, Madison WI) human embryonic stem cell (hESC) line and have followed established serum free embryoid body neural induction methods in combination with dual SMAD inhibition (Noggin and SB431542). For neuronal differentiation we recommend differentiating NSC for at least 3 weeks as the CD44/CD184 double positive glial population requires longer culturing times to differentiate. (Please see Yuan et al. PLoS One. 2011 Mar 2;6(3):e17540 for details). As cells and culturing methods can differ, we recommend a time course analysis to determine appropriate timing for cell sorting experiments.
(All steps performed using sterile techniques)
1. Detach cells of interest from the culture dish. Investigators are encouraged to detach cells at 37°C using Accutase™ Cell Detachment Solution (Cat. No. 561527).
a. If needed some mild trituration may be used to better obtain a single cell suspension.
b. Neuron disassociation may take up to 45 minutes.
2. Collect and spin down cells. Resuspend cells in 10ml of basal w/supplements (e.g. DMEM/F12 + 1X N2 + 1XB27) media containing 100 u/ml DNAse. Incubate at room temperature for 10 minutes.
3. Filter cells through a 70 µm cell strainer and then spin down cells and resuspend cells at 10 million cells/ml in basal media w/supplements and also with 5 mM EDTA + 0.5 percent BSA.
4. Label tubes and add the corresponding antibody conjugates as shown (use appropriate tables listed below for respective cell prep)
a. Use sterile 12x75mm tubes with caps.
5. Add 100 µl of cells to tubes 6 and 7
6. Add up to 1 ml of cells to tube 8.
a. If you wish to sort more than 10 million cells we recommend replicating tube 8 with additional cells that you wish to sort.
7. Incubate cells on ice in the dark for 20-30 minutes.
8. Wash once with 2 ml basal media w/supplements and also 5 mM EDTA + 0.5 percent BSA.
9. Resuspend cells in basal media w/supplements and also 5 mM EDTA + 0.5 percent BSA at a concentration of 2.5 to 5 million cells/ml
a. Alternatively please contact your cell sorter operator to get a suggested final concentration of cells for sorting.
Neural Induction Staining (NSC sort)
Tube label Add (1 test/drop)
1. Unlabeled compensation Negative CompBead plus + Anti-mouse Compbead plus
2. PE compensation Negative CompBead plus + Anti-mouse Compbead plus + CD24 PE
3. PerCP-Cy™5.5 compenstion Negative CompBead plus + Anti-mouse Compbead plus + CD271 PerCP-Cy™5.5
4. PE-Cy7 compensation Negative CompBead plus + Anti-mouse Compbead plus + CD15 PE Cy™7
5. APC Compensation Negative CompBead plus + Anti-mouse Compbead plus + CD184 APC
6. Cells alone Nothing
7. Isotype control Ms IgG1, k PE + Ms IgG1 PerCP-Cy5.5 (CD44 Isotype Control) + Ms IgG1 PerCP-Cy5.5 (CD271 Isotype Control) + Ms IgM PE-Cy7 + Ms IgG1 APC
8. Sort sample CD24 PE + CD44 PerCP-Cy5.5 + CD271 PerCP-Cy5.5 + CD15 PE-Cy7 + CD184 APC
Neuron and Glia Sort
Tube label Add (1 test/drop)
1. Unlabeled compensation Negative CompBead plus + Anti-mouse Compbead plus
2. PE compensation Negative CompBead plus + Anti-mouse Compbead plus + CD24 PE
3. PerCP-Cy™5.5 compenstion Negative CompBead plus + Anti-mouse Compbead plus + CD44 PerCP-Cy™5.5
4. PE-Cy7 compensation Negative CompBead plus + Anti-mouse Compbead plus + CD15 PE Cy™7
5. APC Compensation Negative CompBead plus + Anti-mouse Compbead plus + CD184 APC
6. Cells alone Nothing
7. Isotype control Ms IgG1, k PE + Ms IgG1 PerCP-Cy5.5 (CD44 ITCL) + Ms IgM PE-Cy7 + Ms IgG1 APC
8. Sort sample CD24 PE + CD44 PerCP-Cy5.5 + CD15 PE-Cy7 + CD184 APC
Product Notices
- Please observe the following precautions: Absorption of visible light can significantly alter the energy transfer occurring in any tandem fluorochrome conjugate; therefore, we recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to prevent exposure of conjugated reagents, including cells stained with those reagents, to room illumination.
- PerCP-Cy5.5–labelled antibodies can be used with FITC- and R-PE–labelled reagents in single-laser flow cytometers with no significant spectral overlap of PerCP-Cy5.5, FITC, and R-PE fluorescence.
- PerCP-Cy5.5 is optimized for use with a single argon ion laser emitting 488-nm light. Because of the broad absorption spectrum of the tandem fluorochrome, extra care must be taken when using dual-laser cytometers, which may directly excite both PerCP and Cy5.5™. We recommend the use of cross-beam compensation during data acquisition or software compensation during data analysis.
- This product is subject to proprietary rights of Amersham Biosciences Corp. and Carnegie Mellon University and made and sold under license from Amersham Biosciences Corp. This product is licensed for sale only for research. It is not licensed for any other use. If you require a commercial license to use this product and do not have one return this material, unopened to BD Biosciences, 10975 Torreyana Rd, San Diego, CA 92121 and any money paid for the material will be refunded.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Development References (2)
-
Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009; 27(3):275-280. (Biology: Cell differentiation). View Reference
-
Yuan SH, Martin J, Elia J, et al. Cell-Surface Marker Signatures for the Isolation of Neural Stem Cells, Glia and Neurons Derived from Human Pluripotent Stem Cells. PLoS ONE. 6(3)(Methodology: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.