-
Your selected country is
Middle East / Africa
- Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


.png)

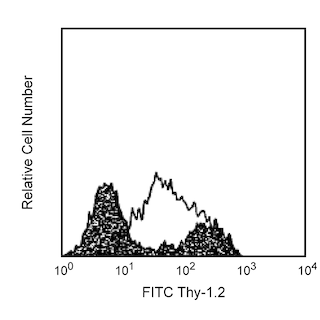
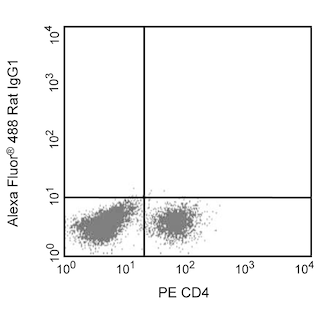
Flow cytometric analysis of intracellular IL-6 expression by activated mouse macrophages. Thioglycollate-elicited mouse peritoneal macrophages were primed with recombinant mouse IFN-γ (10 ng/ml, Cat. No. 554587) for 2 hr and stimulated overnight with lipopolysaccharide (LPS, Sigma, Cat. No. L-8272; 1 μg/ml) and BD GolgiPlug™ Protein Transport Inhibitor (Containing Brefeldin A) (Cat. No. 555029). The adherent cells were washed with 1× phosphate buffered saline (PBS) and incubated with 1× trypsin-EDTA solution (37°C, 15 min). The cells were harvested, washed, incubated with Fc Block™ (Rat IgG2b,κ Anti-Mouse CD16/CD32) antibody (Cat. No. 553142), fixed and permeabilized using BD Cytofix™ Fixation Buffer (Cat. No. 554655) and BD Perm/Wash™ Buffer (Cat. No. 554723). The cells were then stained either with an Alexa Fluor® 488 Rat IgG1, κ Isotype Control (Cat No. 557720, Left Panel) or with the Alexa Fluor® 488 Rat Anti-Mouse IL-6 antibody (Cat No. 561363, Right Panel). MiCK-3 Mouse Cytokine Positive Control Cells (Cat No. 554654) are prepared in a similar manner. These cells can be used as a positive control for cytokine flow cytometry experiments designed to characterize the nature of mouse IL-6-producing cells. Two-color flow cytometric dot plots showing the correlated expression of IL-6 (or Ig Isotype control staining) versus cellular autofluorescence measured in the phycoerythrin channel (autofluorescence) were derived from events with the forward and side light-scatter characteristics of intact macrophages. Flow cytometry was performed using a BD™ LSR II Flow Cytometer System.
.png)

BD Pharmingen™ Alexa Fluor® 488 Rat Anti-Mouse IL-6
.png)
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- Alexa Fluor® 488 fluorochrome emission is collected at the same instrument settings as for fluorescein isothiocyanate (FITC).
- Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- The Alexa Fluor®, Pacific Blue™, and Cascade Blue® dye antibody conjugates in this product are sold under license from Molecular Probes, Inc. for research use only, excluding use in combination with microarrays, or as analyte specific reagents. The Alexa Fluor® dyes (except for Alexa Fluor® 430), Pacific Blue™ dye, and Cascade Blue® dye are covered by pending and issued patents.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products




The MP5-20F3 monoclonal antibody specifically binds to mouse interleukin-6 (IL-6). The immunogen used to generate the MP5-20F3 hybridoma was recombinant mouse IL-6.
Development References (6)
-
Abrams J. Immunoenzymetric assay of mouse and human cytokines using NIP-labeled anti-cytokine antibodies. Curr Protoc Immunol. 2001; 1:6.20-6.21. (Biology). View Reference
-
Abrams JS, Roncarolo MG, Yssel H, Andersson U, Gleich GJ, Silver JE. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992; 127:5-24. (Biology: ELISA, Neutralization). View Reference
-
Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995; 188(1):117-128. (Methodology: Flow cytometry, IC/FCM Block). View Reference
-
Sander B, Hoiden I, Andersson U, Moller E, Abrams JS. Similar frequencies and kinetics of cytokine producing cells in murine peripheral blood and spleen. Cytokine detection by immunoassay and intracellular immunostaining. J Immunol Methods. 1993; 166(2):201-214. (Biology: ELISA, Flow cytometry). View Reference
-
Starnes HF Jr, Pearce MK, Tewari A, Yim JH, Zou JC, Abrams JS. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-alpha challenge in mice. J Immunol. 1990; 145(12):4185-4191. (Biology: Neutralization). View Reference
-
Suda T, O'Garra A, MacNeil I, Fischer M, Bond MW, Zlotnik A. Identification of a novel thymocyte growth-promoting factor derived from B cell lymphomas. Cell Immunol. 1990; 129(1):228-240. (Biology: Neutralization). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.